
ACS PRF | ACS
All e-Annual Reports

44510-AC5
Surface Reactivity of Radicals and Ions During Plasma-Catalytic Removal of Nitrogen Oxide (NOx) Pollutants
The primary focus of this project is to elucidate fundamental chemical processes occurring at catalyst surfaces during plasma-catalytic removal of nitrogen oxides (NOx), beginning with nitric oxide (NO). Mechanisms for NO removal were investigated using the imaging of radicals interacting with surfaces (IRIS) technique, which examines the steady-state reactivity of plasma-generated species at catalytic surfaces. IRIS was also used to complete fundamental relative gas phase density studies and analyze the effects of gas composition, gas additives and applied radio frequency (rf) power.

Gas Phase Density Studies. Initial experiments focused on gas phase density profiles of NO in plasmas by changing gas composition to examine effects of gas additives on NO removal. The relative gas-phase density data reported here represent the average laser-induced fluorescence (LIF) intensity from multiple charge-coupled device (CCD) images, Figure 1, acquired using the same plasma parameters. Error bars represent one standard deviation of the mean.
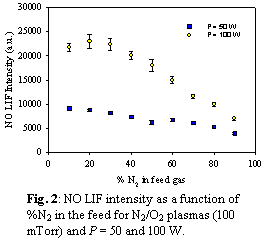
NOx are formed when fuel is burned at high temperatures, the combustion process. However, NOx can also be produced through gas-phase plasma reactions. Figure 2 contains NO LIF data for N2/O2 plasmas as a function of % N2 in the feed. These data were collected at two different applied rf powers (P). For both P, NO concentration decreases as the %N2 increases. There is significantly more NO produced at higher P, however, clearly indicating P dissociates the feed gases, allowing formation of NO.
The amount of N2 in exhaust fumes is ~70%, thus we used this concentration for most of our studies. Because small amounts of NO are found in exhaust, 5 mTorr of NO (5% of total) were used with N2 andO2 (25%). NO LIF signal decreases significantly as P increases, in contrast to the P dependence in Figure 2. This suggests that additional reactions are occurring in the plasma when NO is added to the N2/O2 system. One possibility is that nitrogen and oxygen react with NO instead of each other. Future studies will explore this hypothesis using mass spectrometry and optical emission spectroscopy (OES).
 |
Studies of exhaust systems have shown a water content dependence on NO removal. Thus, we added H2O vapor to our system and monitored the effect on NO concentration. In general, as the humidity increases, the amount of NO removed increases (LIF intensity decreases). At the lowest [H2O], NO appears to be relatively constant with P, which may mean this level of humidity is not sufficient to significantly affect NO removal.
Hydrocarbons are also found in exhaust fumes, thus we also examined effects of methane addition (~3%) on removal of NO. In addition to CH4, the gas mixture also contained N2, O2, and NO. In CH4/N2/O2/NO plasmas, there is a significant reduction in NO as the P is increased, whereas the NO intensity in the (~4%) H2O system does not change significantly with P. This suggests that hydrocarbons may be more efficient than H2O for NO removal.
Identification of excited state plasma species was performed using OES, with Ar added to NO plasmas as a reference. Species identified include NO, N2, O, and Ar. Dependence of OES signals on P was also examined, revealing the same trend as with the LIF studies. Namely, NO density in the plasma decreases with increasing P, Figure 6. Gold coated silicon wafers were placed in the reactor to examine catalytic behavior. At P = 25 W, NO signals were significantly diminished, and at higher P, nearly all of the NO was removed. We are currently exploring plasma-surface interactions of NO using IRIS, and preliminary results suggest NO is highly reactive on a variety of substrate materials.
This project represents a new area of study for the Fisher group as this is the first time we have explored plasma-catalytic processes. Since initiating the project, one graduate student, Ms. Michelle Morgan, has joined the group and focused her studies on NO plasma-catalytic behavior. Currently two new graduate students considering joining the group have expressed specific interest in these projects. Potential additional projects for these students would focus on analysis of other pollutants such as SOx and NO2. During the past year, we have established collaborations with two other groups to obtain catalytic materials. In the future however, we will be synthesizing our own substrates to view the plasma-catalytic effects on a variety of substrates. We would also note that this project has spawned two additional environmentally-related projects in different areas, focused on plasma remediation of dense media (e.g. water) and on modification of materials used in aqueous separations (e.g. desalination).