
ACS PRF | ACS
All e-Annual Reports

44691-G3
New Strategies for Incorporating Magnetic Anisotropy into Single-Molecule Magnets
The synthesis of new single-molecule magnet (SMM) candidate materials requires the balancing of many factors--high nuclearity, magnetic coupling (J), large ground state spin (S) and magnetic anisotropy (D)--most of which compete with each other. The projects undertaken by my group in the first year of PRF support have focused on increasing magnetic anisotropy (D) in exchange-coupled clusters by preparing molecules and ligand systems with topological anisotropy. The hypothesis is that enforcing molecular shape anisotropy will drive an increase in the magnitude of magnetic anisotropy.
(1) Metal-ethynylbenzene chemistry. Although the 1,3,5-triethynylbenzene ligand imposes ferromagnetic coupling between Fe(III) centers in trinuclear assemblies,[1] the ligand has not been used to construct metallodendrimers with paramagnetic transition metal ions. Initial work in our lab concentrated on the synthesis of diamagnetic building block analogues that could be characterized and monitored by 1H NMR spectroscopy. We have prepared both di- and triethynylbenzne ligands (DEB and TEB, respectively), as well as their lithiated, silylated and stannylated analogues, by literature methods. Although acetylides are usually reactive toward metal complexes containing α-hydrogen atoms, we have found that the reaction of trans-[Co(cyclam)Cl2]Cl (cyclam = 1,4,8,11-tetraazacyclotetradecane) with DEB and TEB in methanol in the presence of weak bases yield discrete mono-, di-, and tri-Co(III)-ethynylbenzene architectures 1–4 (Figure 1). These species have been characterized by ES-MS, FT-IR and X-ray crystallography. They are stable in air and in solution.
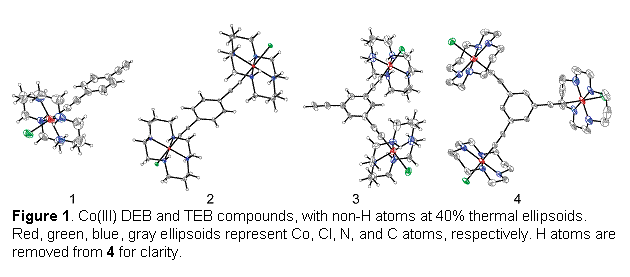
The [Co2] and [Co3] clusters 2 and 4 were studied electrochemically as a possible means for obtaining paramagnetic complexes. The CV experiments show that the reduction of Co(III) to Co(II) is chemically accessible (–1.2 V vs SSCE), however, the clusters dissociate upon reduction.
With diamagnetic building blocks in hand, we turned our attention to paramagnetic analogues. We have undertaken a broad survey of reactions between 1st row transition metal precursors and DEB, TEB and their analogues. Although discrete compounds have not yet been isolated, we observe promising species by ES-MS spectroscopy as well as distinct changes in acetylide stretching frequencies indicative of M-CC bond formation. Efforts to isolate and structurally characterize these species are underway.
(2) Progress toward linear metal-cyanide clusters. The ultimate in topological anisotropy would be attained by synthesizing one-dimensional, linear species. Many magnetic “shish-kebob” coordination polymers have been prepared and studied. Formal truncation of these infinite chains to afford oligomeric assemblies would provide another way to regulating anisotropy.
Scheme 1. Trinuclear cyano-bridged linear complexes.
On this project, I have worked exclusively with undergraduate students. Our short term goal in this project has been to synthesize trinuclear complexes that would show that covalent linkage of Schiff base complexes is an effective way to target linear architectures (Scheme 1). Maximum ligand flexibility can be achieved if the linker species can be attached first, and then condensed with aldehydes to form salophen-type ligands suitable for metalation. We targeted the synthesis of a dicarbamate backbone formed from 1,4-phenylene diisocyanate and 3,4-diaminobenzyl alcohol. DFT calculations indicate that this arrangement of rigid components is sufficiently flexible to bind strongly to a trans-[(salen)M(CN)2] complex. However, our experience and those of others show that aryl isocyanates react with anilines and phenols preferentially to benzylic alcohols. In response, we prepared the benzylic alcohol salophen ligand 8 first (Scheme 2), and metallated with NiCl2 and FeCl2 to produce OH-salophen complexes 9 and 10, using adaptations of literature procedures. These species have been characterized by ES-MS and electronic absorption spectroscopy. Efforts are underway to link 9 and 10 with phenylene diisocyanate.
Scheme 2. Preparation of OH-salophen complexes.

In summary, we have synthesized diamagnetic structural analogues of discotic metallodendrimer building blocks. These species feature trans halide ligands poised for further reactivity and cluster growth. We have also made new metal precursors which could facilitate the synthesis of paramagnetic first generation dendrimers. In addition, we have prepared a series of ligands and transition-metal salophen complexes that are poised for assembly into linear metal cyanide clusters. The elaboration of these species to afford complex transition metal architectures capable of novel magnetic properties is ongoing.