ACS PRF | ACS
All e-Annual Reports

45586-AC3
Tunable Control of Magnetic Properties in Transition Metal Indenyl Complexes
This research has as goals the investigation of the synthesis, structures, and magnetic properties of bis(indenyl)metal complexes that display adjustable changes in spin state. Ultimately we aim to incorporate the compounds into charge-transfer molecular magnets, and create new classes of magnetically active compounds. Some of these may display thermal hysteresis that would be the foundation for data storage applications.
A preliminary study revealed that bis(indenyl) complexes of Cr(II) display spin states that are strongly dependent on the orientation of the indenyl ligands; i.e., compounds with staggered ligands were high spin, with four unpaired electrons (S = 2), whereas complexes with ligands twisted ca. 90° relative to each other were low spin, with two unpaired electrons (S = 1). The centrosymmetry of the staggered complexes prevent the metal d orbitals from interacting with certain combinations of the indenyl's ¹-orbitals, leading to the high spin state. The use of sterically bulky groups such as t-Bu and SiMe3 on the ligands forces them to twist relative to each other, destroying the centrosymmetry. This allows the d orbitals to mix with the ligand orbitals, and generates the low-spin state. We have extended these results to include the intermediate eclipsed conformation, in which the symmetry restrictions on orbital mixing are partially lifted, and whose complexes usually display thermally induced spin-crossover behavior.
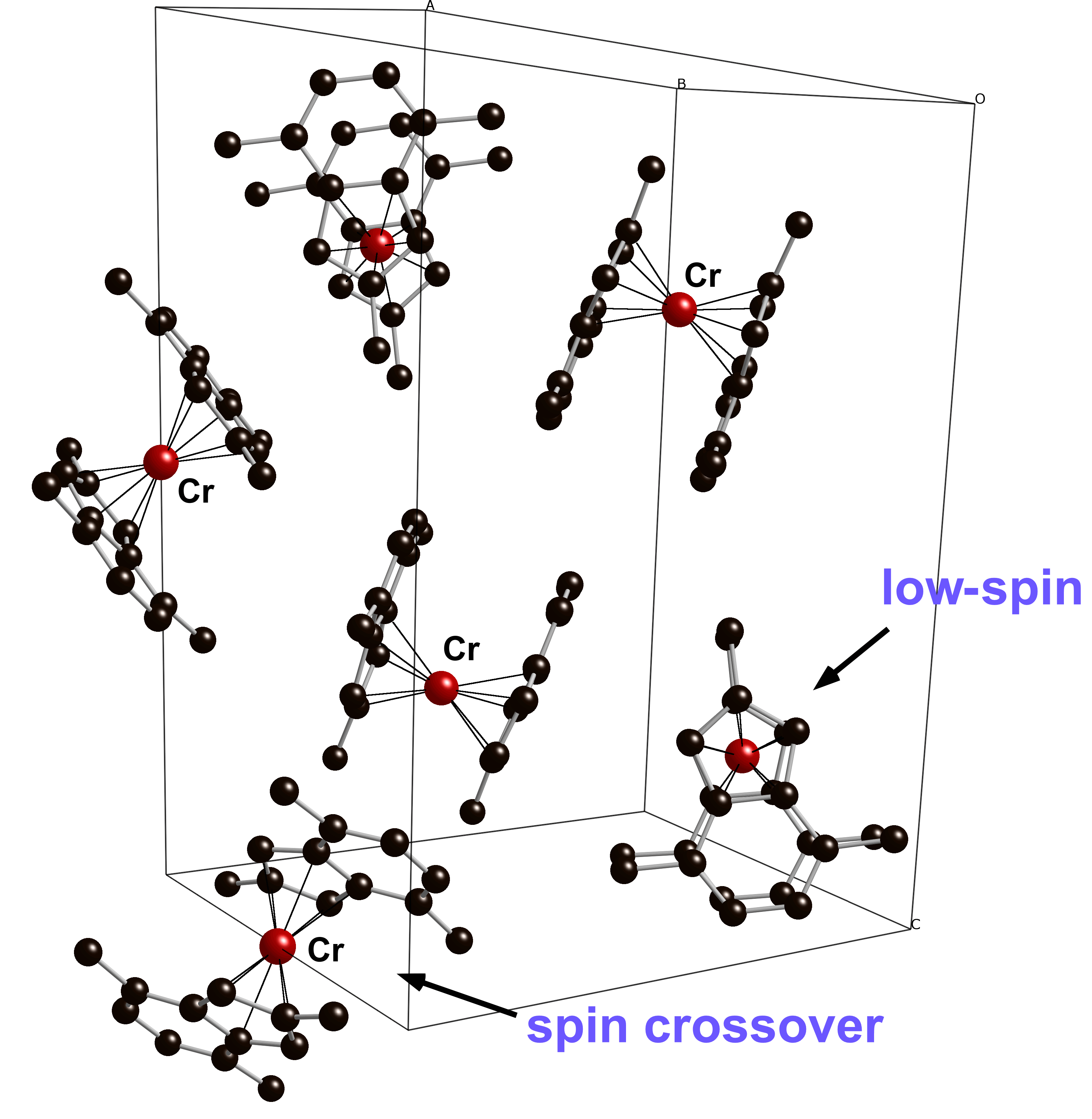
An extensive series of mono- and polymethylated bis(indenyl)chromium(II) complexes has been prepared that display the full gamut of high-spin, low-spin, and spin-cross over behavior. In general, large numbers of methyl groups support low-spin complexes, but their specific location on the indenyl ligands has a strong modulating effect on the spin states; methyl groups on the six-membered benzo ring appear to be somewhat more effective in stabilizing a low-spin ground state than are those on the five-membered ring. The tendency to exist as a low-spin complex is approximately (1,4,5,6,7-Me5C9H2)2Cr > (1,4,7-Me3C9H4)2Cr ~ (4,7-Me2C9H5)2Cr > (1,2,3-Me3C9H4)2Cr > (1-MeC9H6)2Cr ~ (2-MeC9H6)2Cr. Of particular note is the (1,4,7-Me3C9H4)2Cr complex, which crystallizes with two independent molecules in the unit cell, one with an eclipsed conformation, and the other staggered (Figure). On warming from 173 K to 293 K, the staggered form undergoes roughly 3 times the bond length increase as the eclipsed form, which is attributed to the symmetry-driven preference of the staggered configuration for the high-spin state.
We extended our studies to other first row metals, including iron and manganese. In preliminary work, we incorporated (1,2,3-Me3C9H4)2Fe into an air-stable charge transfer salt, [(1,2,3-Me3C9H4)2Fe][DCNQ] (DCNQ = 2,3-dicyanonaphtho-1,4-quinone), and found it to be a metamagnet (i.e., a compound that is antiferromagnetically ordered in zero applied field, but ferromagnetically ordered in a sufficiently high magnetic field) with an ordering temperature of 4.1 K (Figure). The magnetic ordering appears to be a direct result of the matching of the shapes of the indenyl donors and DCNQ acceptors.
The incorporation of complexes with spin-crossover behavior into charge-transfer salt magnets could induce thermal hysteresis. Cationic Mn(III) complexes, containing d4 metal centers, would be isoelectronic with the neutral Cr(II) species, and might display analogous orientation dependent spin crossover behavior. Bis(indenyl)Mn(II) compounds would be the logical precursors to the Mn(III) species. Although the unsubstituted complex (C9H7)2Mn is unknown, we have prepared and crystallographically characterized the THF-stabilized (C9H7)2Mn(thf)2 (which has both η1 and η3-indenyl ligands). Various substituted derivatives, including [2-(SiMe3)2C9H5]2Mn and [1,3-(i-Pr)2C9H5]2Mn (both with staggered ligands) and [1,3-(SiMe3)2C9H5]2Mn (gauche ligands) have been synthesized. All are high spin (5 unpaired e–, S = 5/2) at room temperature. Their redox chemistry is under investigation.
We are continuing to accompany these synthetic, crystallographic, and magnetic studies with computational investigations, mainly employing density functional theory (DFT) methods. We have used these in determining the effect of the location of indenyl substituents on the magnetic behavior of the associated Cr(II) complexes. Preliminary results suggest that the Mn(II) complexes are relatively insensitive to orientation effects; for example, the difference between staggered and twisted (107°) [2-(SiMe3)2C9H5]2Mn is only 3.9 kJ mol–1. Calculations will used to examine the comparative energy changes in the Mn(II)/Mn(III) complexes incorporated into charge-transfer salts.