
ACS PRF | ACS
All e-Annual Reports

42276-AC1
Developing New Electroauxiliaries for the Construction of Template-Based Peptide Libraries
N-acyliminium ions have proven to be very effective tools for both the synthesis of alkaloids1 and the construction of constrained peptidomimetics.2 However, the use of N-acyliminium ions for constructing modified peptides having more than one amino acid is complicated by the need for selectively forming them at only one of the nitrogens in the peptide. This is a problem that we addressed with the use of an silyl-electroauxiliary.2 Electroauxiliaries are groups that lower the oxidation or reduction potential of a neighboring functional group in order to aid electron-transfer reactions.3 In the case of a silyl substituted amino acid, the presence of the electroauxiliary enables the selective oxidation of its amide nitrogen in the presence of other amides (Scheme 1).
Scheme 1
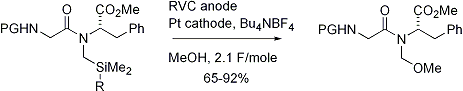
We successfully demonstrated this technique using both electrochemical and chemical oxidations, a series of substituents on the silyl-electroauxiliary, and both di- and polypeptides. However, at the start of the budget period for this project a major problem remained. While the reactions could be used to make simple dipeptide building blocks, the best of the electroauxiliaries (R = 3,5-dimethoxyphenyl) lowered the oxidation potential of the molecule to only +1.3 V vs. Ag/AgCl. This meant that the chemistry was not compatible with a number of amino acid side chains (those containing thiol, phenol, or amine groups). For this reason, we proposed the development of new electroauxiliaries that would further lower the oxidation potential of the system. Although Floreancig and coworkers had demonstrated that lowering the oxidation potential of such systems could interfere with subsequent bond fragmentation reactions,4 we hoped the lability of the silyl group would overcome this complication just as it had with the R = 3,5-dimethoxyphenyl derived case. With this in mind, several new electroauxiliaries were synthesized having amino substituents. In each case, the oxidation potential was significantly lower (+0.5 to +0.8 V vs. Ag/AgCl) than the ones studied earlier, and in each case the oxidation led to a stable radical cation that would not fragment. Clearly, the presence of the silyl group allowed us to push the oxidation potential much lower than previously demonstrated, but the approach was still limited.
What was need was a change in strategy. If an electroauxiliary could not be oxidatively cleaved in the presence on a complex peptide, then maybe it could be used to build a peptide having an N-methoxyalkyl amide that could be used to generate the N-acyliminium ion in a more complex peptide. The use of the electroauxiliary was needed because N-methoxyalkyl amide containing peptides can not be synthesized directly. The starting amino acid is not stable. An example of the electroauxiliary approach is illustrated in Scheme 2. This approach works because of the base stability of the N-methoxyalkyl amide derivative. For example, intermediate 3
Scheme 2

can be saponified, converted into an acitivated ester, and then used in a subsequent coupling reaction, even when that coupling reaction involves placement of the substrate onto the surface of an addressable microelectrode array.5 Once on the array, the microelectrodes can be used to generate acid (an oxidation of diphenylhydrazine) and release the N-acyliminium ion (Figure 1). In the figure the release of the N-acyliminium ion in a checkerboard pattern was detected by exchanging the methoxy group in 4 for 4-pyrenylbutanol and then imaging the chip using a fluorescence microscope.
Figure 1
a) b)


a) Fluorescence image of checkerboard pattern. b) Confinement around a single electrode in the array.
With the development of a strategy for both placing an N-acyliminium ion precursor into a peptide and then releasing the N-acyliminium ion site-selectively on an addressable microelectrode array, work can begin to utilize this chemistry as a tool for the construction of addressable libraries of peptide derivatives. This work is in progress.
References:
1. Maryanoff, B. E.; Zhang, H. –C.; Cohen, J. H.; Turchi, I. J.; Maryanoff, C. A. Chem. Rev. 2004, 104, 1431.
2. Sun H.; Martin, C.; Kesselring, D.; Keller, R.; Moeller, K. D. J. Am. Chem. Soc. 2006, 128, 13761.
3. See Suzuki, S.; Matsumoto, K.; Kawamura, K.; Suga, S.; Yoshida, J. Org. Lett. 2004, 6, 3755.
4. Wang, L.; Seiders, J. R., II; Floreancig, P. E. J. Am. Chem. Soc. 2004, 126, 12596.