ACS PRF | ACS
All e-Annual Reports

43530-GB1
Transition Metal-Catalyzed [3+2] Cycloaddition Reactions Incorporating Carbon Dioxide under Mild Conditions
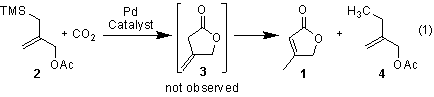
In the previous reporting period, we disclosed the successful Pd-catalyzed reaction of 2-(acetoxymethyl)-3-(trimethylsilyl)propene (2a) and methylated derivatives (2b-2d) with carbon dioxide (Equation 1, Scheme 1) to produce g-butyrolactones (1a-1c). The optimized yield for the conversion of 2a to 1a is 63%. The reactions of 2b-2d were only carried once on a small scale to identify the product regioisomer.
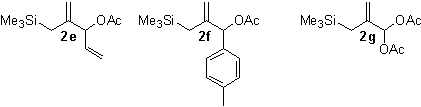
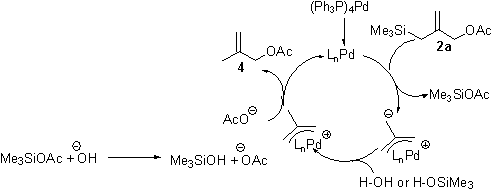
During the past 12 months, we have developed GC and NMR assays to allow rapid and accurate yield determinations of test reactions. We have also carried out preparative scale reactions on substrates 2b-2d, and obtained isolated yields of 46% for the conversion of 2b to 1b, 35% for the conversion of 2c to 1c, and 27% for the conversion of 2d to 1b. Through kinetic studies, we determined that the rate of reaction of substrate 2c is considerably faster than that of the parent substrate (2a), while substrates 2b and 2d (which proceed through a common intermediate) react much more slowly than 2a. All three substrates react faster than the all-carbon [3+2] cycloaddition reported by Trost. Scheme 2. Possible Catalytic Cycle. These data suggest that the attack of the Pd-TMM complex on CO2 is rapid, and the subsequent ring closing through attack of the carboxylate on the p-allyl palladium complex is the slow step in the catalytic cycle (Scheme 2). We synthesized several additional substrates 2e-2g (Figure 1), but to date we have not observed any of the desired [3+2] cycloadducts with any of these substrates; only the protodesilylated starting materials (analogs of 4) were observed. Figure 1. New Substrates for CO2 cycloaddition. It became clear to us that the factor limiting the yield of these reactions is the protodesilylation reaction, so we investigated its mechanism. Based on our experiments, we believe that the Pd-TMM complex is formed rapidly, but then the anionic carbon is protonated by adventitious water (Scheme 3). Scheme 3. Possible desilylation mechanism The hydroxide generated by this process cleaves the trimethylsilyl acetate, and the acetate, then attacks the cationic p-allyl palladium complex. If this mechanism is correct, each water molecule can destroy two molecules of the Pd-TMM complex, which further degrades the yield. Furthermore, substrates 2e, 2f, and 2g have been purified by vacuum distillation into an ice-cold collection flask, and the amount of water that could have condensed into these materials after disassembling the distillation apparatus may very well be enough to effect complete desilylation. We also believe that some water may be present in our carbon dioxide, so we are taking steps to rigorously exclude moisture from our gas and our starting materials in the hopes of suppressing this pathway.