ACS PRF | ACS
All e-Annual Reports

44107-GB7
New Kinds of Responsive Chiral Materials Based on Cholesteric Liquid Crystal Polymers
The goal of the proposal is to create cholesteric polymers that can respond to environmental changes and external chemical agents by changing the pitch of cholesteric helix (and therefore their photonic band gap structure and color) and/or fluorescence. The selectivity of response to different chemicals is supposed to be achieved by modifying the properties of the cholesteric matrix and introducing specific chemical groups that can react or form non-covalent bonds with external chemical agents. This is a challenging task because the introduction of chemically active groups into the cholesteric matrix often suppresses the cholesteric or liquid crystalline state.
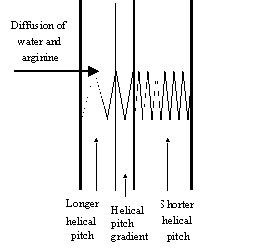
During the first year we concentrated on identifying the extent to which a cholesteric matrix can be modified while retaining its liquid crystalline and chiral properties [1, 2]. Cholesteric polymer films sensitive to the presence of some amino acids (e.g., arginine, lysine, histidine) in water were synthesized by inserting hydrogen bonded chiral dopants and nematic molecules into the cholesteric polymer matrix, which was formed by polymerizing cholesteric liquid crystalline monomer in a planar oriented state [1, 3]. The weakening/strengthening of hydrogen bonds between the polymer matrix's components changes the cholesteric pitch (reflected in color changes and spectral position shifts of the selective reflection band) and the cholesteric material's optical properties. The changes in the hydrogen bonds' strength are achieved by reacting the polymer hydrogen-bonded components with the basic amino acids diffusing from aqueous solutions in which the polymer films are immersed. This process results in a formation of longer helical pitch (see figure). Polymers' sensitivity to amino acids was shown to depend on the polymers composition; higher concentration of hydrogen bonded groups leads to more sensitive polymers.. This effect is explained in terms of the increased hydrophilicity of the polymer matrix and faster diffusion of water into the polymer. Polymers' sensitivity to amino acids may also be amplified by increasing the concentration of chiral compounds, their helical twisting power (their ability to twist the nematic state) and increasing the strength of hydrogen bonding between chiral and nematic molecules (which implies replacing weak hydrogen bonds with stronger hydrogen bonds, for example, replacing hydrogen bonds between carboxylic acids with hydrogen bonds between pyridine and carboxylic acids).
A number of chiral hydrogen bonding molecules are now being screened in order to find those with the highest helical twisting power (HTP). Molecules with the highest HTP will be used to increase cholesteric polymers' sensitivity to amino acids. The interesting consequence of the selective reflection band shift is that optical pumping of cholesteric films doped with laser dyes leads to lasing at different wavelength. Thus the changes in the selective reflection induced by amino acids in water solutions may be detected not only by the naked eye, but also as a laser emission at the different wavelengths [1, 3]. The other approach to increase cholesteric polymers' sensitivity is based on the use of planar cholesteric structures with induced undulations of cholesteric planes [4, 5, 6]. Undulations result from a gradient of helical pitch across the planar cholesteric cell. Even a small gradient in the concentration of chiral molecules may lead to the formation of distorted layers which are clearly visible under a microscope and to the naked eye, and indicate changes in helical twisting power. Thus, diffusion of chiral molecules into the polymer matrix may lead to the formation of ordered undulation patterns that can be easily detected. In order to understand the fundamentals of undulations we used light sensitive chiral molecules as chirality changing agents. Chiral azo compounds undergoing reversible trans-cis-isomerization under UV light were synthesized as light sensitive molecules, changing their helical twisting power and structure. The phenomenological theory that relates the magnitude of light-induced structural changes and cholesteric liquid crystals' material parameters thus was developed. The theory predicts that later undulations' dimensions increase with an increasing ratio between helical twisting power and strength of interaction between chiral molecules and the liquid crystalline matrix. The undulations' size also depends on helical pitch. The theoretical predictions were verified in experiments that opened up an opportunity to create light-tunable chiral lasers. Doping chiral liquid crystals with laser dyes and synthesized light-sensitive azo compounds created a cholesteric photonic band gap structure that emits laser light while being pumped by an external pumping laser. Irradiating this system with UV light shifted the selective reflection band and formed cholesteric undulations that shut down the emission. This result is a surprisingly interesting byproduct of research paving the way to environmentally sensitive polymers. Current research efforts are directed toward creating polymers with frozen (“polymerized”) undulations, which should be sensitive to both chiral molecules and polymers with selective responses to sugars and aliphatic hydrocarbons. We also develop a technique that should allow us to create multylayered structures with interchangeable optical properties and selectivity to aliphatic hydrocarbons and sugars.