ACS PRF | ACS
All e-Annual Reports

44304-GB4
Pyrrole Amides and Their Use as Building Blocks for the Preparation of Anion Receptors
| The objectives of this project are: develop synthetic methodology that allows for the "large-scale" preparation of a diverse library of pyrrole-amides and prepare dipyrrinone analogs containing the pyrrole-amide motif for use as anion receptors. | ||||||||||||||||||||||||||||||||||||||||
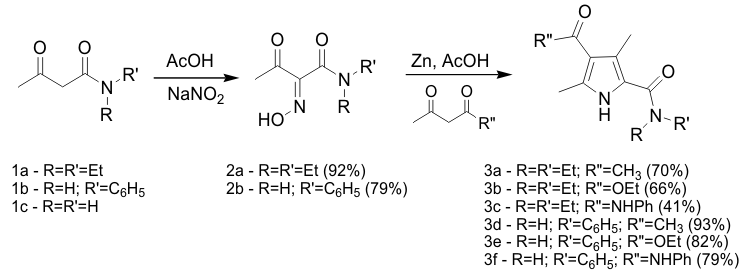
| OBJECTIVE 1 Initial efforts used commercially available acetoacetamide derivatives to probe the versatility of the synthetic route. Reaction 1a and 1b with sodium nitrite in acetic acid gave the corresponding oximes 2a and 2b in high yields. Reaction of oximes 2a and 2b under Knorr conditions with three different beta-keto derivatives resulted in moderate to high yields of the various pyrrole alpha-amide derivatives 3a-f, see Scheme 1. The conditions for both steps of the reaction sequence are amenable to being performed on relatively large scale. | ||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||
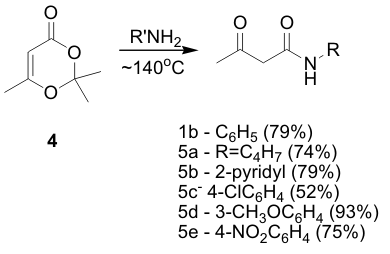
| Achieving diversity of N-alkyl substituents requires the ability to make acetoacetamides with varying N-substituents. Currently, there are several synthetic routes for preparing acetoacetamides in the literature; Meldrum's Acid or diketene (commonly as diketene acetone adduct) are the most commonly used precursors. Reaction of the diketene adduct 4 and various primary amines resulted in acceptable to excellent yields of the corresponding acetoacetamide derivative, Scheme 2. | ||||||||||||||||||||||||||||||||||||||||
 SCHEME 2 SCHEME 2 | ||||||||||||||||||||||||||||||||||||||||
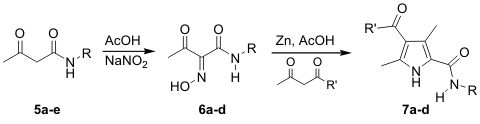
| Most of these acetoacetamide derivatives were easily converted into their corresponding pyrrole amide derivatives 7a-d in respectable yields, Scheme 3. The stepwise preparation of pyrrole 7a resulted in an overall yield of 29% from the acetoacetamide, while the same compound was prepared in 57% overall yield when the crude oxime reaction mixture was used directly in the pyrrole synthesis. Using the acetoacetamides derivatives prepared from diketene, resulted in pyrroles with methyl groups at the 3- and 5-positions. | ||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||
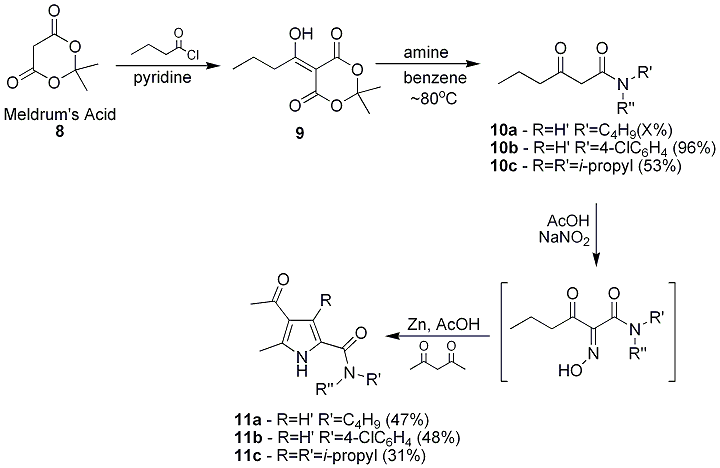
Reaction of the Meldrum's acid, 8, with an appropriate acid chloride followed by addition of a corresponding amine allows for relatively easy preparation of the desired 4-substituted acetoacetamide derivatives. Reaction of Meldrum's acid with butanoyl chloride resulted in the desired acylated Meldrum's acid product, 9, Scheme 4.  Aminolysis of 9 performed resulted in good yields of the corresponding 3-oxo-hexanamide derivatives 10a-c. Using the previously discussed method, the corresponding pyrrole analogs 11a-d were prepared. This work is currently being written up for publication. | ||||||||||||||||||||||||||||||||||||||||
 SCHEME 4 SCHEME 4 | ||||||||||||||||||||||||||||||||||||||||
OBJECTIVE 2 Our initial investigations into the anion binding properties of dipyrrinone amides began with control studies using of 2-ethyl-3-methyl-(10H)-dipyrrin-1-one. This receptor is easily accessible in large quantities in a relatively short overall synthesis. An examination of the concentration dependence of the dipyrrinone NH chemical shifts in CDCl3 and (CDCl2)2 over the temperature range from -20oC to 100oC determined the self-association constant to be 3,850 M-1. Molecular recognition studies have shown that it has a preference for guests with an OH moiety, such as hydrogen sulfate (HSO4-), and carboxylic acids (RCO2H). X-ray structural analysis was also performed on both self-associated dimer and the dipyrrinone tetrabutylammonium hydrogen sulfate complex (See Figure 1 and 2). This work has been accepted for publication in Tetrahedron. | ||||||||||||||||||||||||||||||||||||||||
Table 1. Association Constants (Ka) of 1 in CDCl3 with Various Guest Speciesa
Anions were used as their respective tetra-n-butylammonium salts. Association constant uncertainties estimated at +/-15%. | ||||||||||||||||||||||||||||||||||||||||
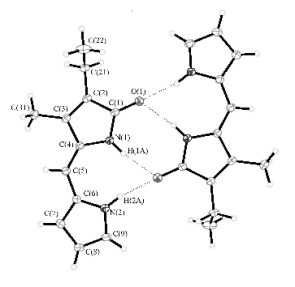 Figure 1. X-ray crystal structure of dipyrrinone showing the dimeric structure. Figure 1. X-ray crystal structure of dipyrrinone showing the dimeric structure. | ||||||||||||||||||||||||||||||||||||||||
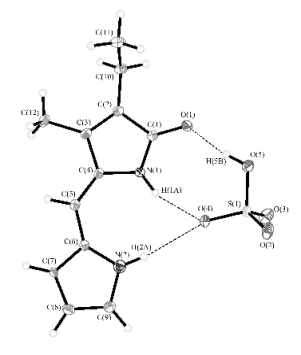 Figure 2. X-ray crystal structure of the dipyrrinone: tetra-n-butylammonium hydrogen sulfate complex. Figure 2. X-ray crystal structure of the dipyrrinone: tetra-n-butylammonium hydrogen sulfate complex. | ||||||||||||||||||||||||||||||||||||||||
| Attempts to prepare the dipyrrinone amide have been hampered by synthetic challenges. However, recent results indicate we may have developed a synthetic method to overcome these challenges. | ||||||||||||||||||||||||||||||||||||||||