ACS PRF | ACS
All e-Annual Reports

40719-GB3
Preparation and Investigation of Terpyridine Containing Polymers with Metal Ions Incorporated into and Pendant to the Polymer Backbone
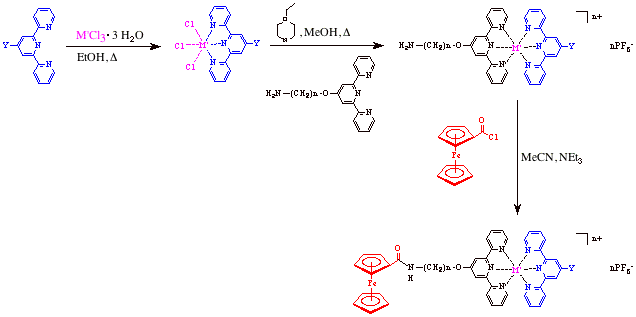
My research group has been investigating the synthesis and characterization of molecular wire candidates that are based on organometallic complexes. We have been successful in preparing small model compounds that contain a ferrocene system coupled to an organic terpyridine bound with a metal salt. This has been accomplished by two different synthetic routes, one of which is outlined in the reaction scheme below. In this reaction scheme a metal salt is initially reacted with an amino-terminated terpyridine in the presence of ethanol. The insoluble metal trichloride salt that was obtained was reacted with another amino-terminated terpyridine ligand that was either the same or different from the initial terpyridine in the presence of N-ethyl morpholine. This led to the isolation of both homo- and hetero-leptic systems. All materials were isolated and analyzed using multinuclear NMR spectroscopy, IR, and UV-vis spectrophotometry. In addition, some of these materials were studied using cyclic voltammetry. Once isolated, these materials were then subjected to a coupling reaction with chlorocarbonylferrocene in the presence of acetonitrile and triethyl amine. This led to the production of the desired target molecules as their hexafluorophosphate salts. Analysis and characterization of these materials was also carried out via NMR, IR, UV-vis and electrochemical methods. Investigations into obtaining high resolution mass spectrometry data are currently underway. Furthermore, these materials are being characterized using elemental analysis. The second synthetic approach to these materials was to begin by carrying out an initial arylation reaction between an amino-terminated terpyridine and chlorocarbonylferrocene in the presence of base to yield compounds with the general formula [CpFe(C5H4C(O)NH(CH2)nO-tpy]. These reactions proceeded with yields between 50-65% and all products were analyzed by multinuclear NMR, IR, and elemental analysis. Once this was complete the substituted product was reacted with RuCl3 hydrate to yield the coordinated compound. The final step was to take this compound and react it with another animo-terminated terpyridine to form the target complex as its hexafluorophosphate salt. Although these reactions took place it was found that solubility issues arose in the final step that caused only small amounts of desired product to form. I had five undergraduate students working on this research project with me this past year, all of whom conducted summer research during the months of May-July 2007. Two of these students had also worked in my research laboratory last summer (2006) and we were able to present our work at the national ACS conference in Chicago this past March. One of these two students is currently working on her honors project with me and we hope to submit a publication to the Journal of Inorganic chemistry or Organometallics by the end of the academic year.