
ACS PRF | ACS
All e-Annual Reports

37447-AC3,1
Reductive Chemistry of Low-Valent Titanium and Zirconium Porphyrin Complexes
Metalloporphyrin mediated reactions provide a variety of useful synthetic transformations. We have shown that low-valent titanium porphyrins are capable of reductively coupling carbonyl compounds to efficiently produce κ2 diolato complexes under an inert atmosphere.1 Treating the Ti(IV) diolato complexes under aerobic conditions resulted in the reverse process, oxidative cleavage of the C-C bond. This led to the development of a catalytic process using O2 that oxidatively converts 1,2-diols to afford ketones and aldehydes using (TTP)Ti=O (TTP is tetratolylporphyrinato). For example, benzopinacole was efficiently cleaved to benzophenone in 98% yield.2
In searching for new catalytic applications of metalloporphyrins, we have found that iron porphyrins are efficient at catalyzing the insertion of carbene fragments from diazo reagents into N-H3 and C-H bonds. This process utilizes an environmentally friendly, commercially available, and relatively inexpensive catalyst and can be easily performed on the bench top without specialized equipment.We subsequently found that iron porphyrins can also catalyze the addition of carbene fragments from diazo compounds to arenes.
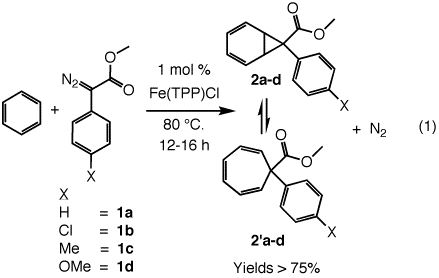
The iron(III) tetraphenylporphyrinato complex Fe(TPP)Cl catalyzes the decomposition of para-substituted methyl 2-phenyldiazoacetates, 1a-d, in benzene to produce rapidly equilibrating mixtures of substituted norcaradiene-cycloheptatriene adducts, 2a-d and 2'a-d (eq 1) in 76-82% yields. This reaction required temperatures of 80 °C for 12 to 16 h with 2 mol% catalyst. No carbene dimerization to form butenedioates was observed. The rapidly equilibrating valence isomers were characterized by 1H NMR and 13C NMR spectroscopy, mass spectrometry and elemental analysis. For example, the formation of 7-carbomethoxy-7-phenylnorcaradiene, 2a, and its ring expansion valence isomer 7-carbomethoxy-7-phenylcycloheptatriene, 2'a, was established by 1H NMR spectroscopy at 20 °C with the appearance of diagnostic resonances for the olefinic hydrogens at 6.28 (m, 2H) and 6.03 (m, 2H) ppm.4 The rapid equilibration of the two species was indicated by the appearance of the 1,6-proton resonance at 4.31 ppm, a position intermediate between those expected for the cyclopropyl hydrogens of static 7-methoxycarbonyl-7-phenyldibenzonorcaradiene (~3.67 ppm)5 and the 1,6-hydrogens of the static 7-carbomethoxycycloheptatriene (~5.43 ppm).6 Furthermore, the 13C NMR spectrum exhibited a broad signal at 72.3 for the 1,6-carbons. This also represented a time-averaged value due to the exchanging vinyl and cyclopropyl carbon atoms at the 1,6-positions. Equilibration of the valence isomers remained rapid even at -60 °C. The composition of the product was verified further by its molecular mass of 226 m/z.
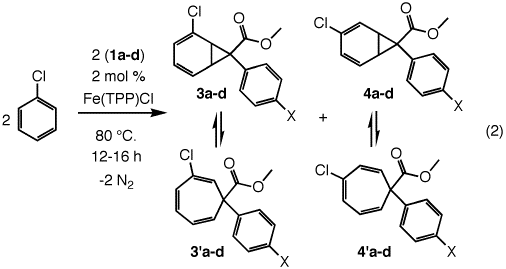
When chlorobenzene was treated with methyl 2-phenyldiazoacetate, 1a, and heated at 80 °C with 2 mol% Fe(TPP)Cl for 16 h, two regioisomers of the fluxional norcaradiene-cycloheptatriene products were produced (eq 2). In all cases, positions 1 and 6 of the valence isomers maintained a C-H group. Thus, the Büchner addition did not involve the chlorinated double bond of the substrate. The ratio of the 2-chloro-products, 3a/3'a to the 3-chloro-isomers, 4a/4'a was 1:1. Separation of the product mixture by silica gel chromatography using hexane/ethyl acetate (30:1) as the eluent produced a pure sample of the 2-chloro isomers, 3a/3'a. The 3-chloro fraction, 4a/4'a, contained some residual 2-chloro products and could not be obtained cleanly. The 1H NMR spectrum of 3a/3'a exhibited signals for the 1,6-protons at 3.30 (dd) and 3.36 (d) ppm respectively. Furthermore, the 13C NMR signal of C7 appeared at 26.2 ppm while C1 and C6 appeared at 43.0 and 44.6 ppm respectively. In contrast, the 1H NMR signals for H1 and H6 of 4a/4'a appeared together at 4.38 (m) ppm while 13C NMR signals for C7 appeared at 38.7 and those of C1 and C6 appeared as broad peaks at 74.7 and 73.5, respectively.
1. Du, G.; Mirafzal, G. A.; Woo, L. K. Organometallics 2004, 23, 4230-4235.
2. Du, G.; Mirafzal, G. A.; Woo, L. K. J. Porphyr. Phthalocya. 2005, 9, 206-213.
3. Baumann, L. K.; Mbuvi, H. M.; Du, G.; Woo, L. K. Organometallics, 2007, 26, 3995-4002.
4. Hall, G.E. ; Roberts, J. D.; J. Am. Chem. Soc. 1971, 93, 2203.
5. Bogdanova, A.; Popik, V. V.; Org. Lett. 2001, 3, 1885-1888.
6. Morilla, M. E.; Diaz-Requejo, M. M.; Belderrain, T. R.; Nicasio, M. C.; Trofimenko, S.; Perez, P. J.; Organometallics 2004, 23, 293-295.