
ACS PRF | ACS
All e-Annual Reports

42115-G1
Synthesis of Homochirally Similar Amino Acids from a Common Synthetic Intermediate
We previously reported our success at using Pig Liver Esterase (PLE) to prepare chiral “half-esters” in good yield and moderate to excellent enantioselectivity. The half-esters contain a chiral quaternary carbon atom and an appropriate side chain of an amino acid. We have made these half-esters which contain side chains of Cysteine, Lysine, Serine, Arginine, Tyrosine, and Isoleucine. The Cysteine, Lysine, Arginine, and Isoleucine half-ester analogues were prepared in >90% ee by PLE hydrolysis. With half-esters in hand we then successfully prepared several unnatural amino acid analogues from the half-ester intermediates in both the d- and l- form. The strategy, using a series of Curtius and Wolff reactions, allowed for the preparation of a-, b2,2-, and b3,3-amino acids from the same common half-ester intermediate.
The isoleucine analogue has a second chiral center in the side chain and we considered several methods to introduce the side chain chiral center. We decided to use isoleucine itself to incorporate the side chain chiral center. Isoleucine was first deaminated to give enantiopure isolvaleric acid which was then converted to the malonic ester common intermediate (Scheme 1).
Scheme SEQ Scheme \* ARABIC 1
|
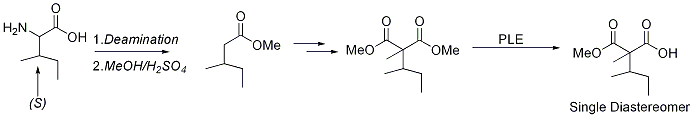
The deamination proceeded in ~80% isolated yield and the esterification was nearly quantitative. The methyl ester was then converted to the malonic ester in ~70% isolated yield using a modification of the Ti enolate chemistry reported by Tanabe1. The PLE hydrolysis was rather slow, but was able to convert the malonic ester to the half-ester intermediate in reasonable yield as a single diastereomer by 1H-NMR analysis. We do not currently know the configuration of newly generated chiral center and we are currently scaling the reaction up to obtain enough material to prove the configuration by synthetic methods.
We are preparing a manuscript for submission to Organic letters detailing the synthetic strategy for the cysteine and serine analogues that we have prepared using this methodology. We intend to submit this manuscript by the end of October 2007. The graduate students working on this project have all given talks over the past year detailing their contributions to this PRF funded project. Two poster presentations were given at the SERMACS meeting held in Augusta, Georgia in November 2006, three talks were given by the graduate students at the Mississippi Academy of Sciences meeting (February 2007), and one poster presentation was given at the National Organic Symposium (Duke University, 2007).
1. Misaki, T; Nagase, R.; Matsumoto, K.; and Tanabe, Y. J. Am. Chem. Soc, 2005, 127, 2854