
ACS PRF | ACS
All e-Annual Reports

44430-G5
Characterization of the Ion Conductivity for Surface Modified Polymer Electrolyte Thin Films
Solid polymer electrolytes are a class of material that has garnered considerable recent attention due to their potential use in polymer electrolyte batteries. More specifically the use of these polymers in lithium ion batteries has been a primary driving force in the research and development of these materials. The use of lithium metal as the anode in these batteries is highly desirable, however over time a passivating or nonconducting layer forms between the polymer electrolyte and the lithium metal rendering the battery useless. Previous work showed that the addition of a self assembled monolayer (SAM) to the polymer surface altered the interfacial properties of the polymer electrolyte, which resulted in a decrease in lithium passivation while maintaining significant ion conduction at the lithium anode [1,2].
The focus of our research in the first year of this grant has been to monitor the growth of a self-assembled monolayer on the surface of a polymer electrolyte as a means to inhibit electrode surface passivation. Our goal was two fold. We wanted to show that SAM growth could be monitored over time using a unique electrochemical arrangement of ac. impedance spectroscopy coupled to an interdigitated microelectrode (IME). We also wanted to see how monolayer growth was altered using molecules with different molecular structures.
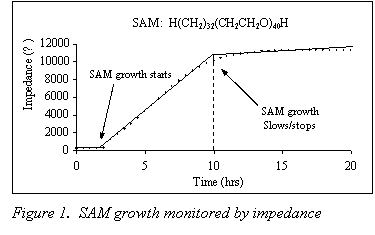
SAM formation was monitored for three different molecules. The molecules had the general formula H-(CH2)32 - (CH2CH2O)y–H, and differed only in the length of the ethylene oxide portion of the molecule ( y = 2, 10 and 40). Figure 1 shows a typical curve obtained for SAM growth on the polymer electrolyte surface. Impedance data showed that the growth times for the three molecules were measurably different from each other, but there was no direct correlation between growth times and molecule size.
To verify the ac. impedance technique as a viable means to monitor SAM growth, attenuated total reflection infrared spectroscopy (ATIR) was used. Figure 2 shows absorption data for the growth of a SAM layer over time. For comparison the molecule shown in figure 2 is the same as in figure 1. It was observed that growth times obtained via IR were similar to those obtained with impedance confirming the use of impedance coupled to the IME as a method for monitoring SAM growth. The results of this work have been accepted for publication [3].

A considerable amount of the reported results are attributed to the efforts of undergraduate student research through our summer research program and the use of this grant. Grant support has also allowed for students to present this work at the National ACS meetings. With this funding support of undergraduate research will continue throughout the course of this work.
[1] R. N. Mason, M. Smith, T. Andrews, and D. Teeters, Solid State Ionics, 118, (1999)
[2] M. Le Granvalet-Mancini, L. Honeycutt and D. Teeters, Electrochimica Acta, 45 (2000) 1491
[3] J. Carlisle and A. Layson, Electrochimica Acta, In press (2007)