ACS PRF | ACS
All e-Annual Reports

43110-GB5
Investigation of Species Related to Silicon Nitride Chemical Vapor Deposition Processes Using Matrix-Isolation Infrared Spectroscopy
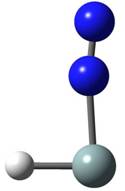
During most of the 2006 - 2007 grant reporting period, we have been performing matrix isolation infrared spectroscopy experiments at 12 K using a vacuum-UV photolysis source with mixtures of silane in nitrogen (SiH4/N2). The specific photolysis source we have been using is a hydrogen resonance lamp, which has a radiative output of 121.6 nm. With the SiH4/N2 hydrogen lamp photolysis experiments, we have observed several new bands that we attribute to transient species. In order to identify the transient molecules formed, we have performed matrix annealing experiments (warming to 30 K), additional ultraviolet-visible photolysis experiments (Hg-Xe lamp), experiments with isotopically substituted reagents (SiD4 and 15N2), and density functional theory calculations (B3LYP/aug-cc-pVTZ). From these experiments and the theoretical calculations, we have identified four transient species containing Si and N2 (Si–N2, Si–(N2)2, HSi–N2, and H2Si–N2) and two transient species containing only Si and H (bridged Si2H2 and trans Si2H4). The HSi–N2 species has not been reported in the literature previously and H2Si–N2 had only been reported once previously but was produced using a different method. The B3LYP/aug-cc-pVTZ optimized structures of HSi–N2 and H2Si–N2 are shown below. The results of the SiH4/N2 H-lamp photolysis experiments have been submitted for publication to the Journal of Physical Chemistry.
HSi–N2 H2Si–N2 We have also performed microwave discharge experiments using SiH4/N2 mixtures at 12 K and appear to be observing infrared bands due to some of the same transient species as were observed in the hydrogen lamp photolysis experiments. The species tentatively identified in the discharge experiments are NH, NH2, N3, N3-, Si–N2, and HSi–N2. Isotopic experiments using SiD4 and 15N2 still need to be performed to confirm the identities of the bands observed. Near the end of the summer of 2007, we began building a pyrolysis source and during the coming academic year we will continue to work on this source. The addition of this source will allow us to examine the type of transient species formed by the application of heat to our reagents. In our future experiments, I plan to investigate the behavior of SiH4 and NH3 mixtures in nitrogen and argon matrices with the hydrogen photolysis lamp to examine if any additional HxSiyNz transient species can be observed. Also, once the pyrolysis source is built, I plan to examine the species formed with this source and the mixtures used in the photolysis and discharge experiments. The financial support of this project by the ACS Petroleum Research Fund in the form of a Type G grant has had a significant impact on the scientific and intellectual development of several undergraduate students at Penn State Erie. With the funds from this grant, I was able to hire one undergraduate student for each of the last two summers to work on the project full time over the summer. Several of the students have now graduated and gone on to positions within the chemistry community and one has gone on to a graduate program. The grant funds have also helped this project by allowing me to purchase some of the chemicals and supplies we have needed, such as several of the isotopic gases, and to begin building our new pyrolysis source, which will give us new opportunities for exploration. The Type G grant has also had a large impact on my own career by allowing me to provide myself with summer salary, thereby enabling me to focus on the research related to the project over the summer months. Additionally, some of the research results obtained from this project have resulted in a submitted manuscript, which will help to strengthen my tenure application.