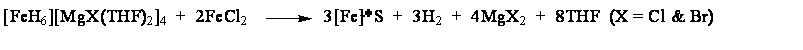ACS PRF | ACS
All e-Annual Reports

41274-B1
Progress on Reaction Chemisty of [FeH6]4-
This past year has resulted in a brief stay in the laboratory of Gregory Kubas (Los Alamos National Laboratory). This was to follow-up on a collaboration that entails the synthesis and characterization of novel complex hydrides of the transition metals.
The reaction that was described briefly in the previous reporting period has been more thoroughly characterized and a submission is currently in print.[1] In essence the solution reaction of [FeH6][MgCl(THF)2]4 in THF with 2 equivalents of FeCl2 results in quantitative formation of metallic iron:
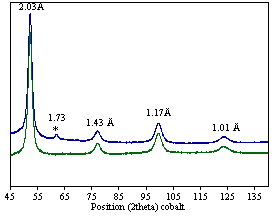
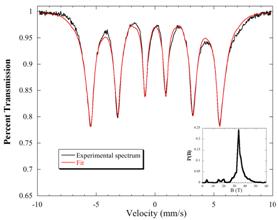
This reaction may be described as solution comproportionation of [FeH6]4- and [FeCl2] to produce principally body centered cubic phase elemental iron, supported by a small amount (16%) magnesium halide, and hydrogen (see Figures 1 and 2 and reference 1). Undergraduates have been involved in studies entailing both the synthetic and quantitative components. Figure 1. XRD of synthesized iron (under Mylar) Figure 2. Refined (NORMOS-90) Mössbauer spectrum Other recent endeavors have also begun to focus on ways to take advantage of the pluripotent [FeH6]4- platform to prepare unique iron hydride complexes supported by cyanide ligands comparable to those known in the case of cobalt(II) cyanides.[2]