ACS PRF | ACS
All e-Annual Reports

42215-AC3
Understanding Radical-Anion-Bridged Coordination Polymer Magnets
In this funding cycle, we have made great progress on the project including the discovery of a rationally designed magnet with a predicted and realized highest Tc in this family of compounds. We have also worked to try to better understand this phenomenon by looking for correlations between the properties of the acceptors and the bulk ordering temperature.
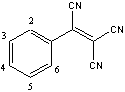 We previously reported that fluorine substituted phenyl tricyanoethylenes one-electron acceptors react with vanadium hexacarbonyl to give three-dimensional coordination polymers with the general formula V[acceptor]2 that order magnetically at relatively high temperatures up to room temperature and beyond. The numbering scheme for substitutions on the phenyl ring is shown at right. The solid is believed to consist of V(II) cations bridged by organic radical anions through the nitrile nitrogen atoms. By varying the substituents on the phenyl ring, we sought to tune the properties of the acceptors.
We previously reported that fluorine substituted phenyl tricyanoethylenes one-electron acceptors react with vanadium hexacarbonyl to give three-dimensional coordination polymers with the general formula V[acceptor]2 that order magnetically at relatively high temperatures up to room temperature and beyond. The numbering scheme for substitutions on the phenyl ring is shown at right. The solid is believed to consist of V(II) cations bridged by organic radical anions through the nitrile nitrogen atoms. By varying the substituents on the phenyl ring, we sought to tune the properties of the acceptors.
acceptor | Tc (K) |
unsubstituted | 215 |
2-fluoro | 259 |
3-fluoro | 238 |
4-fluoro | 159 |
2,3-difluoro | 278 |
2,4-difluoro | 244 |
2,5-difluoro | 276 |
2,6-difluoro | 298 |
pentafluoro | 307 |
Our most impressive result is the magnet created from 2,3,5,6-tetrafluorophenyl tricyanoethylene. Our rationale was that we could surpass the Tc of the previously reported pentafluorophenyl compound by eliminating the fluorine substitution at the 4- position. This goal was realized by starting the synthesis of the desired acceptor with commercially available 2,3,5,6-tetrafluorobenzaldehyde and Tc of the corresponding vanadium magnet was determined to be 315 K, the highest we have observed in this family of compounds.
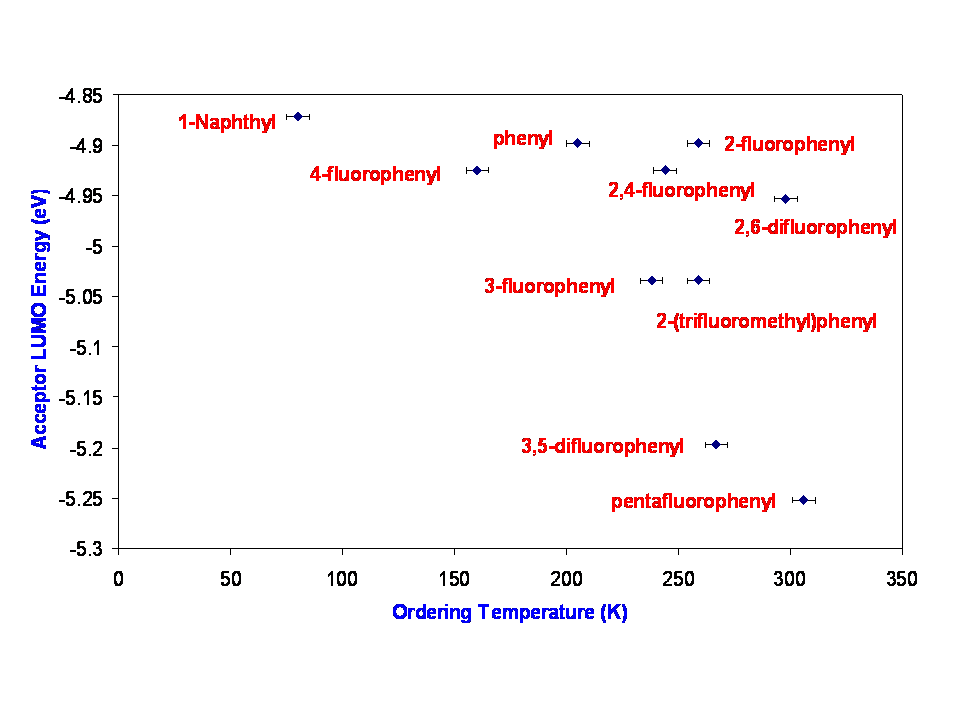
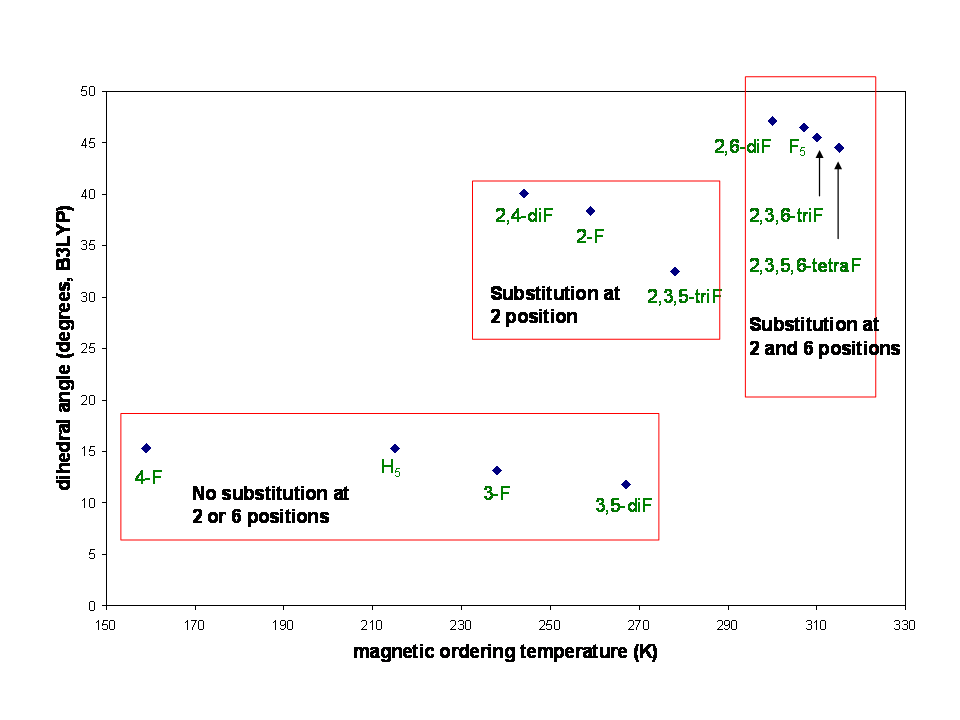
We have also been trying to find correlations between some physical property of the acceptor and the bulk ordering temperature. Such a relationship might be expected to exist since the metal ion is held constant. The two properties that we have examined are 1) the relative energy of the LUMO on the acceptor (as indicated by DFT calculation and electrochemistry; and 2) the dihedral angle formed between the olefin and phenyl ring (as indicated by DFT calculation).
In the Prussian Blue analog magnets, the increase in Tc when incorporating metal ions to the left of the periodic table (V and Cr) has been explained by stronger antiferromagnetic coupling due to higher lying d orbitals in these metals, which better match the energy of the cyanide pi star orbital. A similar argument applied to our magnets would predict that as the energy of the LUMO is lowered (by substitution with electron-withdrawing groups on the phenyl ring), similar stronger antiferromagnetic coupling should ensue and Tc should increase. The LUMO becomes the SOMO upon reduction to the radical anion.
The relative energy of the LUMO was calculated the B3LYP density-functional with the 6-31++G(d,p) basis set within Gaussian-03. Similarly, cyclic voltammetry (not shown) was used to establish the relative energies of the LUMO's. The calculation and the electrochemistry are well correlated, but in each of these cases, although there is a general trend consistent with the hypothesis, (pentafluoro is the easiest to reduce and has the highest Tc) there is very significant scatter in the data that clearly indicates some other effect contributes to the determination of Tc. In particular, the 2,6-difluoro, which gives rise to a magnet with nearly the highest Tc is one of the more difficult acceptors to reduce. A second conclusion from this set of experiments is that there is no obvious electronic reason why substitution at the 4- position is detrimental. The fluorine appears to be electron-withdrawing as expected.
We have also considered whether there is a relationship between the calculated geometry of the acceptor radical anion and Tc and have focused on the dihedral angle formed between the olefin and the phenyl ring, which is strongly affected by substitution at the 2- and 6- positions. What we find is that the greater the angle, generally the higher the ordering temperature is. However, there once again is spread in the data, especially among compounds for which the angle is small. What is most puzzling is that substitutions in the 2- and 6- positions result in loss of conjugation between the olefin and phenyl ring, which seems intuitively undesirable for making it a good acceptor. Thus, if the electronic effects of substitution at the 2- and 6- position are greater assuming flat geometry, those effects are probably somewhat negated by loss of conjugation.