
ACS PRF | ACS
All e-Annual Reports

43014-AC4
Coupled Conformational Equilibria in Peptide-Dendron Conjugates
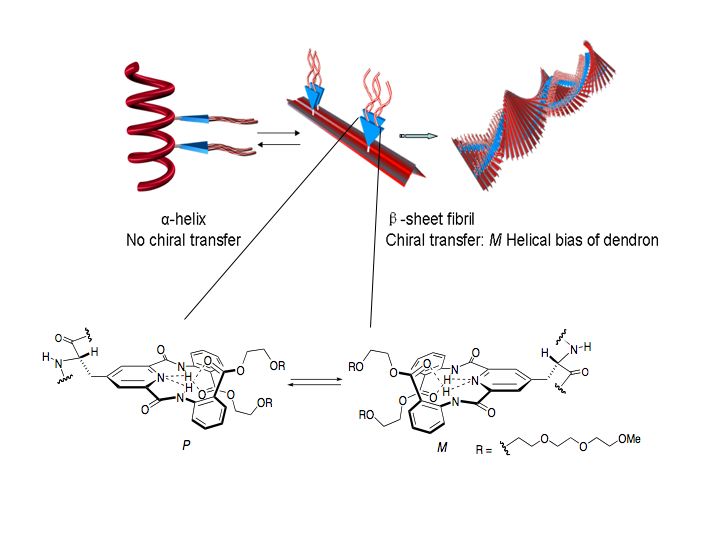 The preprogrammed self-assembly of simple building blocks into higher-order folded domains or complex supramolecular assemblies creates an opportunity to access a diversity of three-dimensional structures and enables a number of applications to be envisaged. The work of this project primarily focuses on understanding how the coupled conformational equilibria of peptides and dendrons in hybrid structures impacts their tendency to form higher-order amphiphilic assemblies. The central hypothesis of this work projected that peptide-dendron conjugates that couple peptide folding/stability with dendron packing would exhibit mutually interdependent conformational equilibria. A series of alanine-rich peptide-dendron hybrids varying the spatial relationship of two dendron-modified amino acid residues, displaying achiral terminal tetraethylene glycol chains, were constructed using solid-phase techniques. All intradendron spacings except the (i, i+6) and (i, i+10) spacings afforded a-helical structures that increased in helicity at higher TFE/PBS ratios, exhibiting maximal a-helical contents of ca. 50% at 60 % TFE/PBS, slightly lower than that of the control peptide lacking any dendrons. Peptide-dendrons with (i, i+6) and (i, i+10) intradendron spacings adopted b-sheet structures. An intense negative couplet centered at ca. 316 nm, indicating an M helical bias in the dendrons, emerged for the b-sheet forms of (i, i+6) and (i, i+10) peptides in PBS, but disappeared in 60% TFE/PBS (where the peptides are monomeric a-helices) and was absent in PBS and 60% TFE/PBS for all the other peptides. Atomic force microscopy of both peptides (AFM) revealed amyloid-like nanofibrils in PBS buffer at 5-10 mM, whereas in 60% v/v TFE/PBS globular aggregates were observed. Accordingly, fibrillogenesis was completely reversible by changing solvent. Therefore, the hypothetical relationship between dendron packing and peptide-dendron conformational synergism was fulfilled within a b-sheet context wherein hydrophobic dendron packing induces a conversion from an a-helical to a b-sheet structure that displays strong chiral communication lacking in the a-helical form. These studies point to the importance of controlled self-assembly (of b-strands into b-sheet fibrils) for chiral communication and; conversely, structures lacking peptideˆdendron chirality transfer do not form stable b-sheet fibrils.
The preprogrammed self-assembly of simple building blocks into higher-order folded domains or complex supramolecular assemblies creates an opportunity to access a diversity of three-dimensional structures and enables a number of applications to be envisaged. The work of this project primarily focuses on understanding how the coupled conformational equilibria of peptides and dendrons in hybrid structures impacts their tendency to form higher-order amphiphilic assemblies. The central hypothesis of this work projected that peptide-dendron conjugates that couple peptide folding/stability with dendron packing would exhibit mutually interdependent conformational equilibria. A series of alanine-rich peptide-dendron hybrids varying the spatial relationship of two dendron-modified amino acid residues, displaying achiral terminal tetraethylene glycol chains, were constructed using solid-phase techniques. All intradendron spacings except the (i, i+6) and (i, i+10) spacings afforded a-helical structures that increased in helicity at higher TFE/PBS ratios, exhibiting maximal a-helical contents of ca. 50% at 60 % TFE/PBS, slightly lower than that of the control peptide lacking any dendrons. Peptide-dendrons with (i, i+6) and (i, i+10) intradendron spacings adopted b-sheet structures. An intense negative couplet centered at ca. 316 nm, indicating an M helical bias in the dendrons, emerged for the b-sheet forms of (i, i+6) and (i, i+10) peptides in PBS, but disappeared in 60% TFE/PBS (where the peptides are monomeric a-helices) and was absent in PBS and 60% TFE/PBS for all the other peptides. Atomic force microscopy of both peptides (AFM) revealed amyloid-like nanofibrils in PBS buffer at 5-10 mM, whereas in 60% v/v TFE/PBS globular aggregates were observed. Accordingly, fibrillogenesis was completely reversible by changing solvent. Therefore, the hypothetical relationship between dendron packing and peptide-dendron conformational synergism was fulfilled within a b-sheet context wherein hydrophobic dendron packing induces a conversion from an a-helical to a b-sheet structure that displays strong chiral communication lacking in the a-helical form. These studies point to the importance of controlled self-assembly (of b-strands into b-sheet fibrils) for chiral communication and; conversely, structures lacking peptideˆdendron chirality transfer do not form stable b-sheet fibrils.