ACS PRF | ACS
All e-Annual Reports

42229-G4
Epsilon-Purine Amino Acids in Sequence-Specific, Self-Folding Architectures
I. Overview
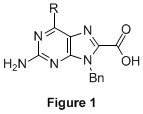
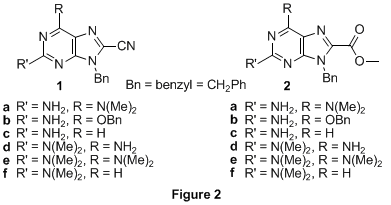
Single-stranded, sequence-specific oligomers that adopt conformationally ordered states in solution play unique roles in chemistry and biology. We originally proposed to prepare a new class of self-folding oligomers based on a heterogeneous peptide backbone, in this case the result of linking novel epsilon-purine amino acids (Figure 1) with conventional alpha-amino acids. The work is part of a long term goal to endow synthetic oligomers with increasingly complex functions. Over the past 12 months we have made additional building blocks toward this goal and rigorously characterized them in terms of their photophysical properties; those studied over the lifetime of this grant are shown in Figure 2 as 1a-f and 2a-f. The molecules, what we are calling quintessential donor–acceptor purines, are among the most highly photoluminescent nucleobases known in organic solvents (and even water); a portion of the work has recently appeared in print. Preliminary studies show that epsilon-purine amino acids are accessible from 1 and 2, and that these intermediates can be converted to amides using routine coupling reagents/conditions. Future work will see the incorporation of the fluorescent units into synthetic peptide architectures with unique optical and self-folding properties in solution.
II. Research Progress We reported last time on the synthesis of D-A purines a–d; we have since expanded the list to include e and f. The protocol takes C(2) and C(6) donor substituted purines that are brominated at C(8) and then transforms them via a palladium(0)-catalyzed cyanation reaction to their corresponding nitriles. Conversion of the nitriles to methyl esters comes by treatment with acidic methanol. The C(8) acceptor groups dramatically increase the fluorescence quantum yields of the purines (versus a hydrogen in the C(8) position) in organic solvents to in many cases near unity (for 1b, a quantum yield enhancement of > 2500% in methylene chloride upon conversion of C(8)-H to C(8)-CN). Absorption (lambda max 310-365 nm), emission (lambda max 360-465 nm), and fluorescence lifetime data (8 ns ≥ tF ≥ 0.5 ns) were collected for all 12 D-A purines in four organic solvents (dioxane, methylene chloride, DMSO, and methanol) and even water (for the 2-aminopurine analogues). The largest red shifts and quantum efficiencies are observed when the strongest donor (N(CH3)2) is on C(2); competitive donors on C(6) reduce the quantum yield substantially for the nitriles. The solvent studies are new and have revealed some expected (e.g. general Stokes shift dependence on solvent polarity) and some unexpected trends. For nearly all of the purines, the quantum yield decreases with increasing solvent polarity (although much less so for the esters). In contrast, 1c and 2c show quantum yield increases with increasing solvent polarity (to near unity in water). Three of the purines have been studied in the solid state by X-ray crystallography, 1a, 1d, and 2d. All show characteristic hydrogen bonding patterns and dipolar pi-stacking (distance between purine planes ~ 3.3-3.4 Å); the ester derivative 2d, for example, also reveals self-complementary dimers held together by four hydrogen bonds. The solid-state patterns suggest ways that the molecular recognition properties of the purines might be used to control bulk ordering and device (e.g. OLED) performance. Theoretical calculations (DFT and ZINDO/CI) have determined the absorption spectra, HOMO (-6.5 to -5.5 eV) and LUMO (-2.5 to -1.5 eV) energies, and the ground and excited state dipoles. The calculated orbital energies are in good agreement with solution-phase cyclic voltammetry data and the calculated excited state dipoles (~ 11 D for 1d) agree well with results from a Lippert-Mataga treatment of the solvent-dependent emission data (~ 14 D for 1d). Initial studies show that amide bonds can be formed at the purine C(8) position (by coupling the intermediate carboxylic acid with a primary amine using DCC/DMAP) toward use of the molecules as the intended heterocyclic amino acids. The amides appear to show high quantum yields (quantum yield > 0.85) in all tested organic solvents. III. Broader Impacts The work described here, made possible through a PRF Type G grant, represents the combined efforts (over two years) of one graduate student, several undergraduates, and one high school student (through the UF Student Science Training Program). The graduate student recently obtained her Ph.D. (8/2007) in chemistry from the University of Florida. During her tenure she became an expert in the synthesis of purine heterocycles and their associated photophysical characterization. For the PI, PRF funding has led to the scientific results required for longer-term support of this research and provided the infrastructure for training students in the areas of synthetic and physical organic chemistry.