
ACS PRF | ACS
All e-Annual Reports

43494-B10
Hybrid Inorganic-Organic Conducting Nanocomposites
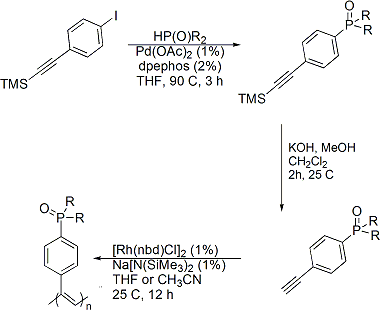
During the second year of the project, the researchers focused on preparing the polymers that will serve as the solid supports for the construction of the new hybrid materials. The basic approach that was followed entailed generation of the functionalized monomers followed by polymerization. The functional groups targeted in this work were phosphoryl donors, with common sources of these groups being phosphine oxides and phosphonates. These donors were of interest for this work since they are known to form strong bonds to lanthanides and actinides. The polymers successfully prepared by functionalization of existing monomers are summarized in the figure below. While several substrates and polymers (poly-1 and poly-2) were prepared using microwave assisted hydrophosphinylation reactions, these polymers were quite insoluble in common organic solvents and molecular weight determinations were only possible on low molecular weight fractions (Mn<50K). To increase the processability of the materials and the scope of the project, alternative monomers were prepared bearing longer alkyl chains. These precursors were prepared using hydrogen phosphonates and secondary phosphine oxides as substrates. Through palladium catalyzed cross coupling reactions, the corresponding precursors for poly-3, 4, 5, 6 were generated. Polymerization of these functionalized monomers using rhodium based catalysts in acetonitrile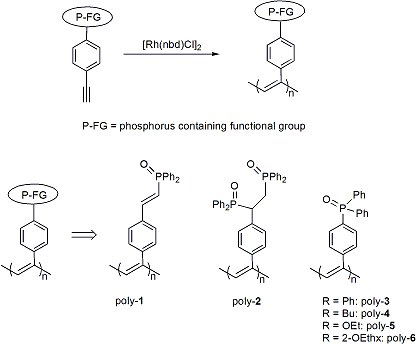
=
=
The physical properties of poly-3, 4, 5,6) are quite different from poly-1 and poly-2. For example, while poly-1 and poly-2 are quite insoluble in polar and non-polar solvents, poly-3 is partially soluble in THF and NMP, and poly-4, 5, and 6 are quite soluble in a range of common solvents. Additionally, poly-1 and poly-2 are weakly emissive (blue) in the solid state, whereas, the remaining monomers and polymers are not emissive.A range of phosphoryl containing polymers were successfully prepared through initial functionalization of a commercially available monomer followed by polymerization using rhodium-based catalysts. The final phase of the project will focus on the generation of hybrid materials using the polymers listed above as solid supports.