
ACS PRF | ACS
All e-Annual Reports

42087-AC5
Studies of the Interfaces between Conjugated Oligomers and Self-Assembled Monolayers
Significance of this Project
This PRF-supported research is aimed at studying interfacial electronic properties of conjugated oligomers deposited on top of organic layers, including self-assembled alkanethiols and buckminsterfullerene. Organic-organic interfaces are critical for new generations of organic electronic devices. The conjugated oligomer chosen for our initial investigations is sexithiophene (6T) because of its use in organic field-effect transistor and photovoltaic devices. In addition to exploring the interfacial electronic properties, we have demonstrated that patterned alkanethiols may be used as templates to guide the subsequent deposition of a soluble form of 6T.
Summary of Progress
Deposition of sexithiophene (6T) on octadecanethiol (ODT) and 1H,1H,2H,2H-perfluorodecanethiol (PFDT) alkanethiol monolayers self-assembled on gold surfaces has been carried out in ultrahigh vacuum. For comparison, thiophene monomer has been adsorbed at 130 K by leaking thiophene vapor into the vacuum chamber. X-ray and ultraviolet photoelectron spectroscopies (XPS and UPS) are used to measure the interfacial electronic properties. In the case of PFDT, the binding energy of the F 1s XPS peak decreases following thiophene and 6T adsorption, indicating charge transfer to the fluorinated SAM layer. UPS measurements confirm this, with the valence features of thiophene and 6T in contact with the monolayer appearing at higher ionization energies compared to thicker layers. Knowledge of how the vacuum levels align is crucial for organic electronic devices because it allows prediction of overlap of the filled and unfilled electronic states between different layers and the efficiency of energy transfer. The energies of the UPS-measured vacuum levels of 6T deposited on PFDT/Au illustrate the absence of a common vacuum level between the organic layers at the PFDT-6T interface and the presence of a -0.9 eV interface dipole. Similar measurements performed for 6T deposition on self-assembled ODT show a weaker +0.4 eV interface dipole. The relatively large value and sign of the 6T/PFDT/Au interface dipole suggest that charge transfer to the PFDT-covered surface results in the formation of dipoles with their negative ends toward the Au surface, in contrast to the 6T-ODT interface. The effects of a SAM layer on X-ray-induced oligomerization, which is known to facilely occur, were also investigated. Comparison of the thickness of oligomeric thiophene formed by X-ray irradiation on clean and PFDT-covered gold surfaces demonstrates that a thicker oligomer layer forms on the SAM-covered surface, suggesting that the spacing provided by the monolayer reduces quenching of electronic excitations that lead to X-ray-induced oligomerization.
A method of patterning a dibutylphosphonate-substituted, soluble form of 6T with nanoscale lateral dimensions has also been developed in a joint effort between this PRF project and an NSF project. Briefly, this method consists of using either microcontact printing or dip-pen nanolithography to form hydrophilic alkanethiol patterns on a gold surface. The remainder of the surface is backfilled with a hydrophobic alkanethiol. When the soluble 6T is spin-coated and annealed, it preferentially adsorbs on the hydrophilic patterns. This patterning method may be useful for future nano-scale device fabrication, and we have demonstrated the patterning of parallel 50 nm wide conjugated oligomer lines. Furthermore, by using substituted silanes instead of alkanethiols, it should be possible to pattern conjugated oligomers and polymers on silicon oxide surfaces.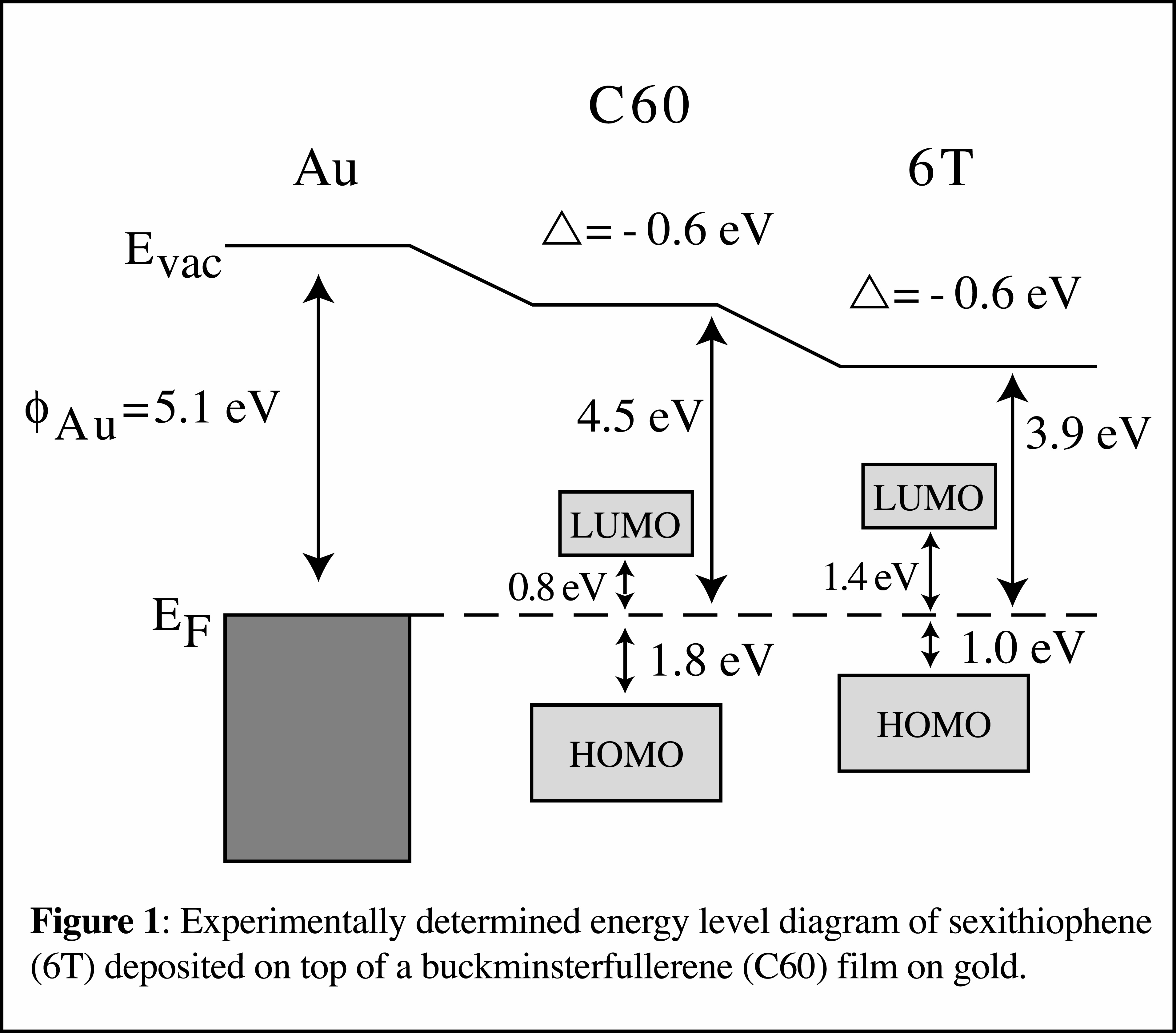 Experiments were also carried out regarding deposition of 6T on top of buckminsterfullerene (C60) films. Photovoltaics may be fabricated by blending (or co-evaporating) C60 with conjugated polymers and oligomers, including 6T. In these devices, C60 serves as an electron acceptor, and the conjugated polymer or oligomer serves as an electron donor. In our PRF-supported research, UPS and XPS have been used to investigate the interface between thermally deposited C60 and 6T films on gold surfaces. Charge transfer from 6T to C60 is indicated by shifts in the interfacial C60 (6T) highest occupied molecular orbitals toward lower (higher) ionization energies. An absence of vacuum level alignment with an offset of 0.6 eV ± 0.05 eV is observed, independent of the deposition order. The vacuum level offset matches the difference in work functions between the C60/Au and 6T/Au surfaces. This demonstrates that the vacuum levels of the organic layers remain pinned to the Fermi level, even though an intermediate organic layer exists between the gold surface and the top organic layer. The energy level diagram for the case of 6T deposited on top of a C60 layer on gold is shown in Figure 1. The energies of HOMOs were determined from UPS experiments in our laboratory, and the energies of the LUMOs were estimated from literature inverse photoelectron spectroscopy data.
Experiments were also carried out regarding deposition of 6T on top of buckminsterfullerene (C60) films. Photovoltaics may be fabricated by blending (or co-evaporating) C60 with conjugated polymers and oligomers, including 6T. In these devices, C60 serves as an electron acceptor, and the conjugated polymer or oligomer serves as an electron donor. In our PRF-supported research, UPS and XPS have been used to investigate the interface between thermally deposited C60 and 6T films on gold surfaces. Charge transfer from 6T to C60 is indicated by shifts in the interfacial C60 (6T) highest occupied molecular orbitals toward lower (higher) ionization energies. An absence of vacuum level alignment with an offset of 0.6 eV ± 0.05 eV is observed, independent of the deposition order. The vacuum level offset matches the difference in work functions between the C60/Au and 6T/Au surfaces. This demonstrates that the vacuum levels of the organic layers remain pinned to the Fermi level, even though an intermediate organic layer exists between the gold surface and the top organic layer. The energy level diagram for the case of 6T deposited on top of a C60 layer on gold is shown in Figure 1. The energies of HOMOs were determined from UPS experiments in our laboratory, and the energies of the LUMOs were estimated from literature inverse photoelectron spectroscopy data.