ACS PRF | ACS
All e-Annual Reports

42614-AC3,10
Microporous Metal Organic Materials (MMOMs) as Hydrogen Storage Media
The last several years have witnessed enormous efforts in developing alternative and renewable energy sources/carriers that are clean and abundant. Seeking for effective hydrogen storage materials is an important ongoing research as part of such efforts since hydrogen could be one of the most ideal alternative energy carriers meeting these requirements.
Microporous metal organic frameworks (MMOFs) represent a new type of sorbent materials that show promise for hydrogen storage. Recent studies on these remarkable materials have uncovered many unique and interesting properties. As a subclass of MOFs these three-dimensional framework structures contain micropores (pore diameter less than 20Å). While having a similar physisorption mechanism as other porous materials, such as zeolites and single-walled carbon nanostubes (SWNTs), MMOFs possess several highly desirable features that may lead to enhanced MOF-H2 interactions. For example, the MMOFs incorporate a variety of metals and ligands that can be activated and functionalized, and thus, are likely to interact with H2 more strongly; The MMOFs contain perfectly ordered pores that allow hydrogen to access the interior space more effectively; The crystal structures and pore properties of the MMOFs can be systematically and deliberately modified to achieve higher hydrogen binding energy. Furthermore, the synthesis is simple, highly reproducible and cost-effective.
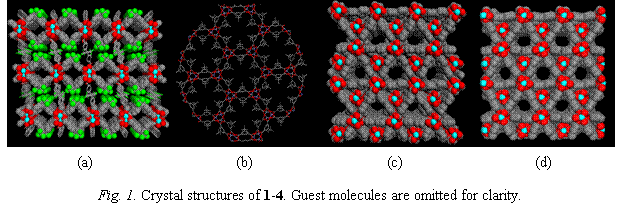
In this work, we have successfully designed and synthesized a number of MMOF materials. We have characterized their crystal and pore structures and properties, and evaluated their hydrogen adsorption capability. Our initial investigations focused on two guest-free metal organic frameworks (GFMMOF) that are exceptionally stable but have smaller pores. [Cu(hfipbb)(H2hfipbb)0.5](1) (H2hfipbb = 4, 4'-(hexafluoroisopropylidene) bis(benzoic acid)) is built on bimetallic Cu2 paddle-wheel units. It is a 3D network having 1D open channels with a pore diameter about 3.5-4.5Å (Fig. 1a). The most striking feature of this compound is its unique shape and size selectivity for hydrocarbons. Compound 1 has a very small pore volume, 0.03 cc/g. It adsorbs only 0.21 and 0.23 wt% of H2 at 87 and 77K (1 atm), respectively. GFMMOF [Zn(tbip)](2) (H2tbip = 5-tert-butyl isophthalic acid) was obtained with similar pore size (~4.5Å) but its 1D channels are more effectively packed, and thus, giving a higher pore volume (0.7 cc/g). The crystal structure of 2 contains tetrahedral zinc metal centers that are linked by tbip ligands to form a 3D framework (Fig. 1b). A 0.75 wt% and 0.52 wt% of hydrogen uptake was achieved at 77K and 87K and 1 atm, respectively. This compound also exhibits a unique capability of separating methanol (MeOH) from dimethylether (DME) or from water.
By use of longer ligands, two highly porous structures [M3(bpdc)3bpy]·4DMF·H2O [M = Zn (3) and Co (4)] were synthesized. Both structures exhibit two-fold interpenetration which gives rise to 1D pores (Fig. 2c-d). The estimated pore volumes are 0.33 and 0.38 cc/g for 3 and 4, respectively. Significantly improved hydrogen uptakes, 1.74% (1.32%) and 1.98% (1.48%) for 3 and 4 at 77 (87) K and 1 atm, were achieved. The PRF grant has provided direct financial assistance to two postdoctoral associates and three graduate students in their research projects. It has enabled the PI and her research group to explore and to develop an entirely new research area in hydrogen adsorption in microporous materials. The research in this area is critical not only for a better understanding of the MOF-H2 interactions and adsorption mechanisms, which will in turn guide our future designing of MMOF materials with significantly improved functionality and performance, but also for making a closer connection between academic research and industrial applications in gas storage and separation.