ACS PRF | ACS
All e-Annual Reports

43238-AC1
Sequential Birch Reduction-Allylation/Cope Rearrangement for the Enantioselective Construction of Carbocyclic Quaternary Stereogenic Centers
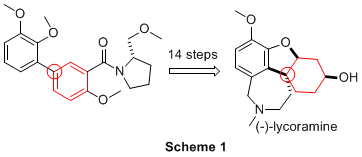
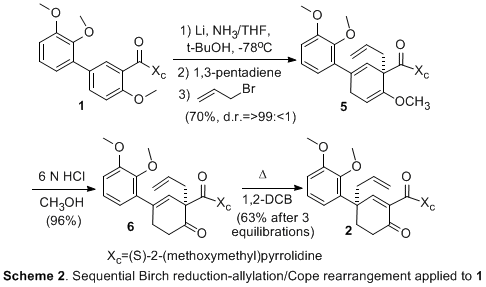
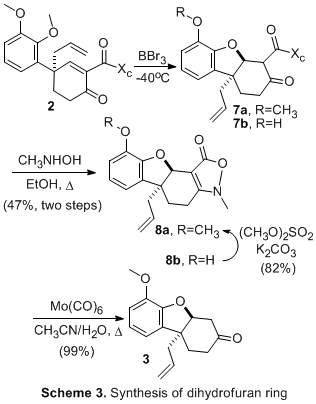
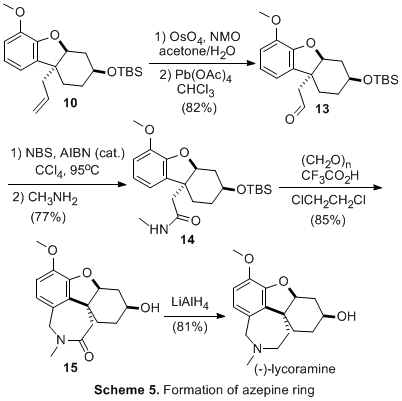
The first enantioselective synthesis of (-)-lycoramine was achieved in 14 steps and 5% overall yield from the biaryl derivative 1 (Scheme 1). The synthesis applied the previously developed Birch-Cope sequence (Scheme 2) to create the key arylic quaternary stereocenter of (-)-lycoramine with excellent enantioselective control. The product of the Birch-Cope sequence, a versatile 4,4-disubstituted-2-carboxamide-2-cyclohexen-1-one 2, was elaborated through an intramolecular conjugate addition of a phenol to create 3, which has the dihydrofuran ring of (-)-lycoramine (Scheme 3). Stereoselective reduction of the ketone in 3 (Scheme 4) was followed by chemoselective elaboration of the allyl group into amide 14 (Scheme 5). Following literature precedent for a racemic lycoramine synthesis, the azepine ring was formed by a modified Pictet-Spengler reaction and reduction of the amide. A crystallized sample of the final product had identical properties to the values reported for the natural product. This achievement accomplishes the fifth and final goal in the original PRF proposal.
Scheme 1.
Scheme 2. Sequential Birch reduction-allylation/Cope rearrangement applied to 1. Scheme 3. Synthesis of Dihyrofuran Ring. Scheme 4. Stereoselective Formation of Alcohol. Scheme 5. Formation of Azepine Ring. The activities of the past year were undertaken by the principal investigator, William P. Malachowski; a post-doctoral fellow, Tapas Paul; and two undergraduate research assistants, Sophia Phounsavath, and Iva Yonova. The principal investigator has benefited tremendously with a significant increase in research productivity as a result of the resources, both personnel and supplies, obtained through PRF funding. In addition, the principal investigator spent much of his sabbatical completing the synthesis of (-)-lycoramine. The post-doctoral fellow worked on the grant for two of the twelve months and, during this time, he was able to hone valuable skills in enantioselective synthesis and natural product total synthesis. He gained greater independence in his ability to conduct synthetic chemistry research in an academic environment, i.e. a greater knowledge of experimental and analytical techniques. In November 2006, the post-doctoral fellow moved to a new synthetic post-doctoral position for a different synthetic chemistry experience. It is his long-term goal to find a full-time position in the pharmaceutical industry and I believe that he made strong progress toward this goal. The undergraduate researchers have likewise been exposed to methodology development in the field of asymmetric synthesis. They have been involved in the synthesis of a complex natural product and, in the process, undergone significant growth in their ability to conduct synthetic chemistry research. They have learned a range of synthetic techniques from reaction preparation and set-up to product purification and analysis. One of the students (Sophia Phounsavath) has graduated and is currently enrolled as a chemistry graduate student at In conclusion, all five goals in the original research proposal have been accomplished. We continue to pursue the application of the Birch-Cope sequence and related synthetic tools to the efficient creation of complex bioactive molecules and we plan to apply to the National Institutes of Health to obtain further funding to continue this line of research.