
ACS PRF | ACS
All e-Annual Reports

43878-G7
A Novel Methodology for the Synthesis of Main-Chain Chiral Polymers
ACS Petroleum Research Fund Annual Progress Report Introduction
A selective and mild chemical modification of polymer can yield a new class of functional materials in a convenient manner. Especially, creation of chiral functionality (e.g., chiral alcohol and amine) along the main-chain of polymer is expected to bring unprecedented interesting properties. Utilizing recent prolific development in asymmetric catalysis, the PI proposed to create optically active alcohols and/or amines in the polymer main-chain by enantioselective reduction of polyketones and/or polyketimine derivatives.
During the first year of the proposed period, we were focused on synthesis of monomers that will be used for preparation of precursor polymers (see Scheme 1). Several bromophenyl ketone derivatives (1M, 1P, 2M, 2P, 4M, and 4P) were synthesized from their corresponding meta– and para–bromobenzaldehyde via reaction with the Grignard reagents followed by oxidation with pyridinium chlorochromate (PCC). Among them, 2M and 2P were unstable under the oxidation condition and isomerized to more stable conjugated enones 3M and 3P, respectively. Structures of those monomers were confirmed by 1H and 13C NMR spectroscopy and GC-MS. Because 3M and 3P do not contain terminal alkene moiety which is required for the polycondensation coupling by Heck reaction, only monomers 1M, 1P, 4M, and 4P were used for the next polymerization step.
Scheme 1. Synthesis of monomers.
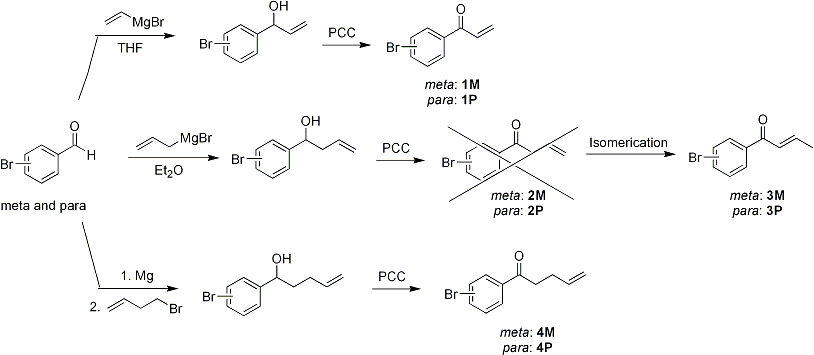
Palladium-catalyzed Heck reaction of the each monomer (1M, 1P, 4M, and 4P) was screened using different palladium sources, ligands, solvents, and temperatures. Although all monomers were polymerized to produce polymers, but their molecular weights measured by size exclusion chromatography using THF as eluent were so far not high (Mn = 3–5 kg/mol). Thus, we are continuing to optimize the polymerization condition to obtain higher molecular weight (Mn > 10 kg/mol).
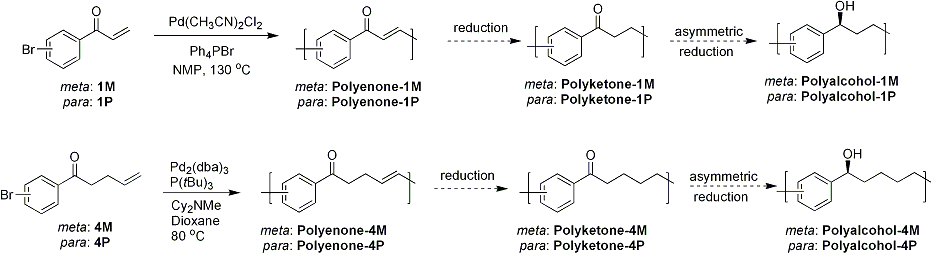
Once we synthesize high molecular weight polyenones, the alkene moiety of those polymers will be reduced using p-toluenesulfonyl hydrazide to give their corresponding polyketones, the immediate precursor polymers of chiral polymers. Finally, asymmetric reduction of the ketone group in the polymer main-chain will generate optically active alcohol functionality along the polymer chain. Once they are synthesized, the optical properties of the polymers will be investigated.
Scheme 2. Synthesis of precursor polymers and future plans of chiral main-chain polymers.
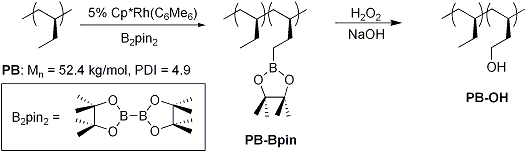
In a related study, we successfully found a way to introduce hydroxy group to the side-chain of isotactic poly(1-butene) via rhodium-catalyzed C–H activation/borylation followed by oxidation of the boronic ester group. This side-chain hydroxylated polymer will be investigated as a new chiral polymer. Based on the 1H NMR spectrum of the hydroxylated polymer, up to 10 mol% of side chains was found to be functionalized with hydroxy group. This new functionalization of saturated crystalline polyolefin will allow creation of a new class of functionalized polymer materials based on polyolefins, inexpensive bulk materials.
Scheme 3. Incorporation of hydroxy group to saturated polyolefin.
The research project will advance discovery and understanding for the synthesis of chiral polymers. In addition, the project will provide the following benefits: (a) comprehensive research training on organic chemistry, polymer synthesis, and job opportunities for students in the PI's research program, and (b) strengthening of the vibrant and growing organic functional materials research program of the PI's lab and improving research infrastructure at University of Nevada Las Vegas. Due to the multidisciplinary nature of the research program, students working under this project will be exposed to different areas of chemistry (i.e., organic, organometallic, and polymer chemistries) and material characterization. During the summer of 2007 a graduate student and several undergraduate students were involved this project. Exposing those students to the various fields of chemistry mentioned above will equip them as a good problem-solving researcher in the future.