
Back to Table of Contents
43594-AC4
Transient Species in Supramolecular Solids
Philip Coppens, State University of New York (Buffalo)
Following our time-resolved studies of the formation of ms-lifetime excimers of the metastable dianion [CuI(NH3)2]22+ and of the aromatic molecule xanthone, both embedded in supramolecular solids (as described in the previous Progress Report), we have studied isomerization reactions which proceed within host-framework structures. The ultimate goal of the work is to identify systems suitable for determination of transition state geometry by ultrafast time-resolved diffraction techniques. We find that, as in the case of the photo-excited triplet states studied, the molecular framework acts as a scaffold that holds the crystal together notwithstanding the change in shape of the embedded guest molecules in the course of the chemical reaction. Such single-crystal-to-single-crystal reactions are rare in neat crystals and in that case mostly limited to monomolecular cyclizations. A total of three studies have been completed:
1. Single-crystal-to-single-crystal E→Z photoisomerization of tiglic acid (2,3-dimethylacrylic acid).
This topotactic reaction occurs in the supramolecular crystals of CECR·HTA. ·2MeOH·1.5H2O (1) (HTA = tiglic acid, CECR = C-ethylcalix[4]resorcinarene) The photostationary state is reached at a concentration of ca 30% of the Z isomers. A kinetic analysis shows the Z→E back-reaction to proceed with a larger rate constant than the E → Z isomerization.
2. Single-crystal-to-single-crystal E → Z and Z → E photoisomerizations of 3-chloroacrylic acid (HClA). In this case both the supramolecular solid incorporating the Z isomer and that containing E
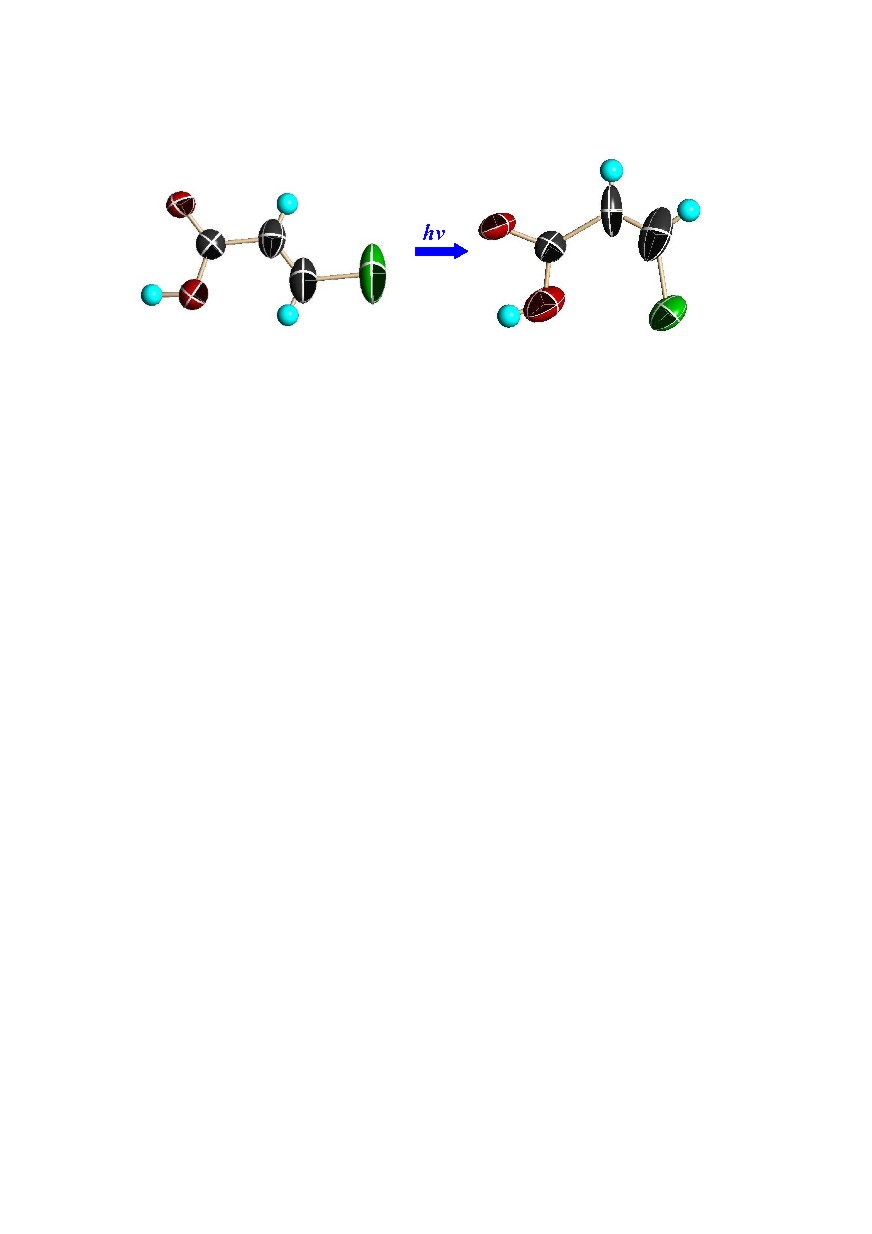
Fig. 1. Perspective views showing 50% probability displacement ellipsoids of the E-HClA before exposure and the Z-HClA after 1 hours exposure.
can be synthesized as isomorphous crystals with composition CECR·HClA·2MeOH·1.5H2O, so that a photostationary state can be approached from two different starting points. The reaction is illustrated in Fig. 1.
The two photostationary states do not have the same composition (Fig. 2), a difference attributed to different softness of the reaction cavities, which differ in expansion on isomerization. The kinetics of the reaction are obtained from the diffraction-determined composition of the crystal after different light-exposure times. The forward and backward reactions in both solids show first order kinetics, but the rate constants differ, as is evident from Fig. 2.
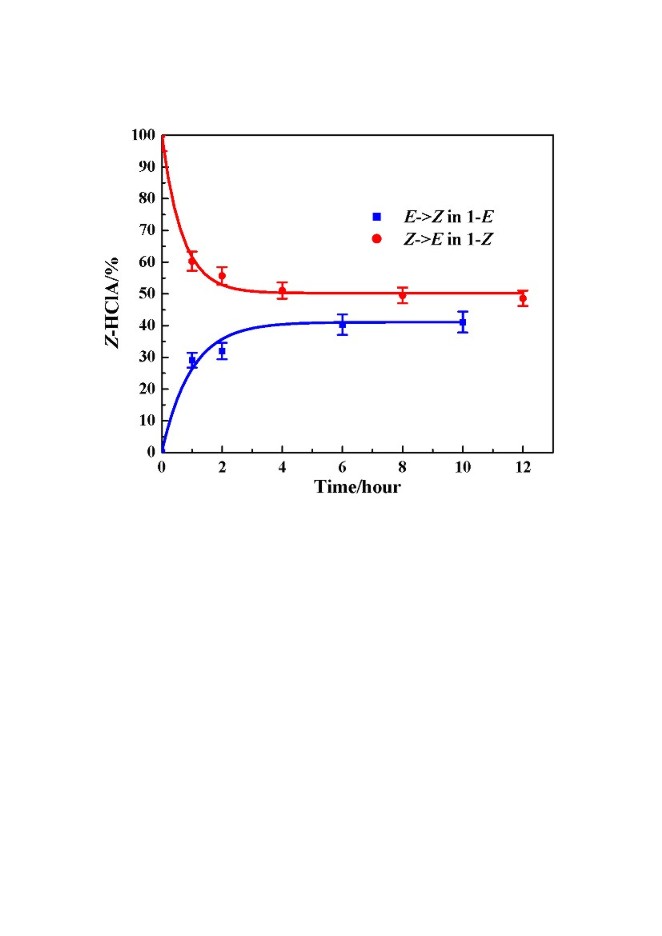 Fig. 2: Percentage Z-HClA in the CECR host-guest complexes as a function of irradiation time.
Fig. 2: Percentage Z-HClA in the CECR host-guest complexes as a function of irradiation time.3. Isomerization of tiglic acid coordinated to a metal atom in a supramolecular solid. As in the other solids studied the photoisomerization of tiglic acid in CECR·[Zn(TA)2(H2O)2]·4H2O proceeds without the [2+2] dimerization reaction that often occurs in crystals of uncomplexed analogues. During the reaction the coordination of the Zn atom is reduced from 5-fold to 4-fold. The reaction is illustrated in the photodifference map shown in the synopsis. The two Zn coordinated TA molecules are located in cavities of very different size (100 and 150 Å3 respectively) (Fig. 3). The rate constants of the isomerization reaction are strongly affected by the size of the reaction cavity. Analysis of the temperature dependence of the reaction rates with Arrhenius and Eyring plots (Fig. 4) shows the activation energies and the standard enthalpies of activation to be dependent on the difference between the volume of reaction cavities. This is the first quantitative diffraction study of solid-state E/Z isomerization of a metal-coordinated ligand in a periodic host environment.
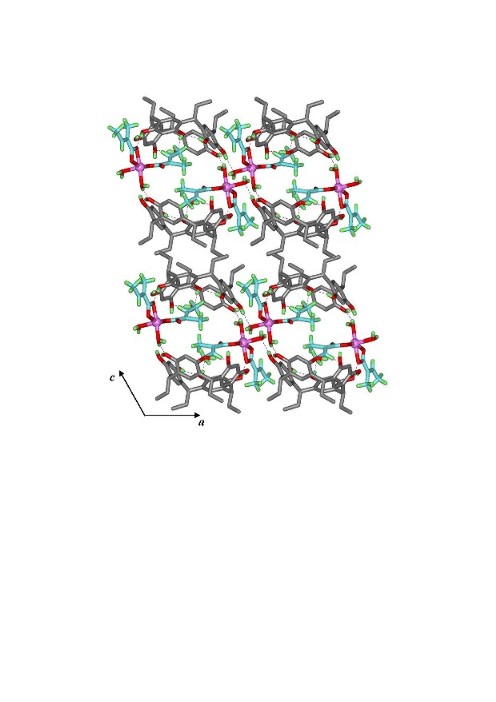
Fig.3. Structure of CECR·[Zn(TA)2(H2O)2]·4H2O viewed along the b-axis direction (Zn atoms displayed as a spheres). The tiglic acid in the small cavity is interspersed between two CECR host molecules.
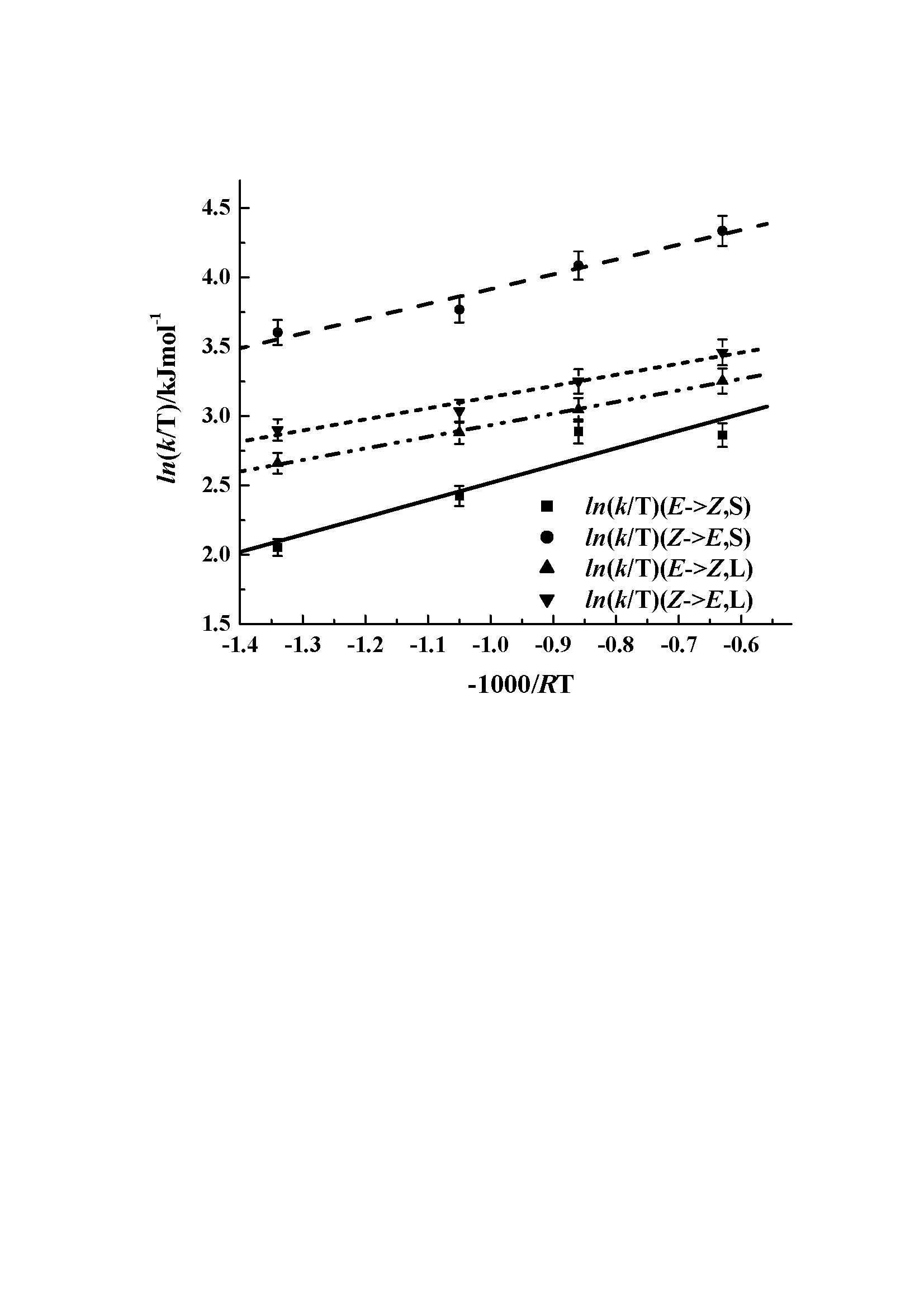
Fig. 4. Eyring plot of the isomerization rates as a function of temperature (EàZ,S, S=small cavity, solid line; ZàE,S, dashed line; EàZ,L, L=Large cavity, dash-dot-dot line; ZàE,L, line with short dashes).
Back to top



 Fig. 2: Percentage Z-HClA in the CECR host-guest complexes as a function of irradiation time.
Fig. 2: Percentage Z-HClA in the CECR host-guest complexes as a function of irradiation time.
