ACS PRF | ACS
All e-Annual Reports

45312-B3
Microwave-Promoted Assembly of Organometallic Hosts: Responsive Systems for Small Molecule Binding
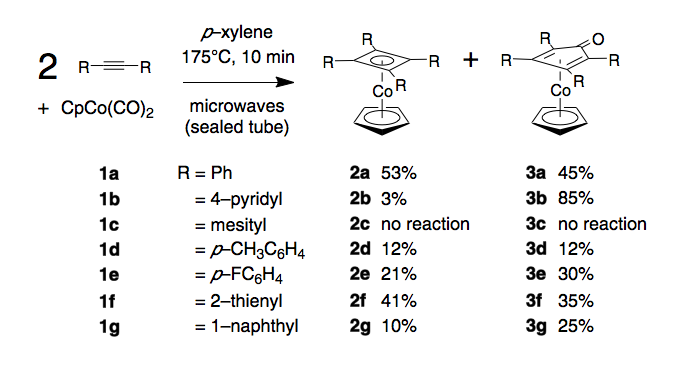
Throughout the past year we have continued to explore the synthetic scope and utility of our approach to the construction of eighteen–electron cobalt metallocenes under microwave conditions. Through a series of experiments designed to delineate the upper and lower steric limits of the synthesis we have been able to demonstrate that a wide variety of diaryl acetylenes 1a–g may be successfully employed to obtain, in generally good yield, both known and novel metallocenes, 2a–g and 3a–g. While the outcome of some of these reactions are known from traditional thermal syntheses, typically a twenty–four hour reflux in a high boiling solvent, our immensely convenient approach typically affords higher yields. In certain cases, most notably for use of the dinaphthyl acetylene 1g, our approach provides the only viable route to preparation of the product complexes. The upper steric limit in this series is reached in the case dimesitylacetylene, a material which fails to yield any metallocene products. This result suggests that non–planarity in the parent diarylacetylene is a crucial factor in determining the overall efficiency of complex production.
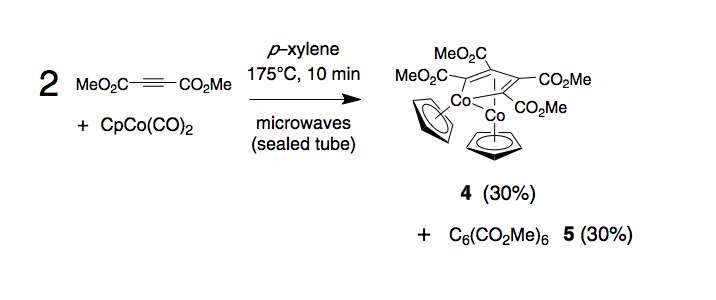
The successful preparation of several new cobalt complexes in these series prompted the question of whether a non–aryl substituted acetylene could be successfully employed in complex formation. Dimethyl acetylenedicarboxylate was selected for its versatility as a dienophile in cycloaddition reactions, and because the cobalt source used in our reactions, CpCo(CO)2, is known to be an effective catalyst for cyclotrimerization of this species. However, by running the reaction at a relatively low ratio of acetylene to CpCo(CO)2 we aimed starve the reaction of the acetylene required for efficient cyclotrimerization and capture those organometallic species favored under these conditions. Microwave irradiation of mixtures of dimethyl acetylenedicarboxylate and CpCo(CO)2 under the standard microwave conditions gave a 30% average yield of dinuclear cobalt complex 4 as the sole isolable organometallic product. The only other identifiable material was the product of cobalt–mediated cyclotrimerization of the starting acetylene, hexamethyl benzenehexacarboxylate 5, also isolated in an average yield of 30%. No evidence for formation of metallocenes of type 2 or 3 was found. Complex 4 is air and light stable and high quality single crystals were readily obtained by evaporation of a hexane solution. The structural formulation proposed in the scheme, while wholly consistent with 1H and 13C NMR data, was proven with this X–ray analysis. Compound 4 joins a small number of dinuclear complexes of this class that have previously been prepared and studied, in part because of their relevance to the mechanism of acetylene cyclotrimerization. As dicobalt complex 4 appears to represent a stable intermediate on the pathway to benzene derivative 5 it appeared unavoidable that addition of further acetylene, followed by further microwave irradiation, should engender further production of 5. Addition of two equivalents of dimethyl acetylenedicarboxylate to the crude reaction mixture containing 4 (obtained from an initial 10 minute microwave reaction), followed by 10 minutes further microwave irradation, prompted further production of 5 (Å30%), while the amount of complex 4 held essentially constant. This outcome supports the view that formation of significant amounts of complex 4 is a result of limiting the amount of acetylene available, and further suggests that a range of acetylenes might be usefully exposed to 4 to achieve assembly of a range of heavily substituted benzene derivatives. The extent to which ready access to complex 4 also provides a versatile entry point to consider mechanistic issues also remains to be explored.