
ACS PRF | ACS
All e-Annual Reports

44507-GB7
Design of Biodegradable Surfactants to Control and Manipulate Physical Properties of Polymeric Nanoparticles Made by Emulsion Evaporation Method
The ultimate goal of the present research is to design bio-friendly surfactants (BFS), derivatives of alpha-tocopherol, to be used in the synthesis of biocompatible and biodegradable nanoparticles of controlled physical properties (size, morphology).
More specifically, the following objectives were proposed:
- To synthesize bio-friendly surfactants by covalently linking ascorbic acid to alpha-tocopherol derivatives
- To synthesize PLGA nanoparticles using the emulsion-evaporation method in the presence of the new, bio-friendly surfactants.
- To characterize the PLGA nanoparticles in terms of size, size distribution, morphology, and toxicity.
Of the three objectives, objective 1 was met during 2006-2007, as anticipated. A bio-friendly surfactant was synthesized from alpha-tocopherol, ascorbic acid, and maleic anhydride. Solubility, surface tension in water, and antioxidant activity of the surfactant were determined, and new research opportunities were opened, as described in the following paragraphs.
Bio-friendly surfactant (BFS) synthesis
Most effort was spent on the synthesis of the BFS. The BFS was made following the synthetic pathway shown below. The final structure was confirmed by C-NMR.
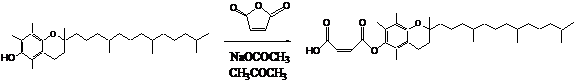
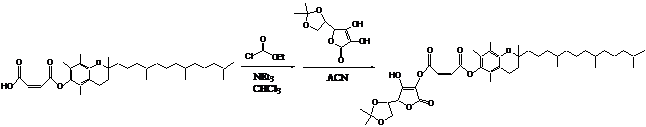

|
Bio-friendly surfactant (BFS) HLB and surface tension activity
The calculated hydrophilic/lipophylic balance of the BFS was 9.2 (on a 0-40 scale, with 40 as most hydrophilic), which suggested that the water solubility of the component was very low; this was confirmed experimentally (solubility of the BFS in water was measured as 0.25 mM). Increasing concentration of BFS reduced the surface tension of water from 77 mJ/m2 for pure water to 66 mJ/m2 at 0.23 mM of BFS.
BFS antioxidant activity
Antioxidant activity of the BFS component was measured by the DPPH method (Table 1). Vitamin E and vitamin C were used as controls.
Table 1. Antioxidant activity o f BFS as compared to alpha-tocopherol, ascorbic acid, and a 1:1:1 molar mixture of alpha-tocopherol:ascorbic acid:maleic acid (N=3)
Component | MW (Da) | TEAC (mM) | IC50 (mg/ml) | IC50 Literature values (mg/ml) |
a-tocopherol | 430.71 | 0.982 | 8.39 | 12 (Han et al., 2004) |
Ascorbic acid | 176.12 | 0.916 | 3.38 | 5.2 (Miller et al., 2001) |
BFS1* | 685 | 5.007 | 56.78 | N/A |
Mixture** | 240.980 | 1.445 | 5.98 | N/A |
TROLOX | 250.3 | 1.000 | 4.29 | N/A |
* BFS1= L-ascorbic acid-2-O-maleic acid-alpha-tocopherol diester
** Mixture= 1:1:1 molar mixture of ascorbic acid (C), alpha-tocopherol (E) and maleic acid (M)
The TEAC is defined as the concentration (mM) of a compound that results in the same inhibition percentage as a 1 mM solution of Trolox (the hydrophilic homologue of alpha-tocopherol). The smaller the TEAC value, the higher the antioxidant capacity of the component of interest. Ascorbic acid (TEAC=0.916 mM) performed slightly better than alpha-tocopherol (TEAC=0.982 mM). An equimolar mixture of alpha tocopherol, ascorbic acid, and maleic acid had a TEAC of 1.445 mM. In comparison, the three components chemically linked to form the BFS component showed a higher TEAC value of 5.007, which implies that to obtain an antioxidant performance similar to that of alpha-tocopherol, a concentration of BFS roughly five times higher was necessary (Table 1).
These results are not surprising, considering that the phenolic –OH of the alpha-tocopherol was not available as a H+ donor following BFS synthesis, and thus the antioxidant capacity of alpha-tocopherol was lost. Ascorbic acid also participated with an –OH during synthesis, and thus the resulting component had a TEAC value smaller than that of free ascorbic acid. Nonetheless, the new surfactant did exhibit antioxidant properties which we may be able to improve in the future (see below).
Future plans
The synthesis of a new surfactant with improved surfactant properties is the objective of the second year. The new surfactant will be synthesized from alpha-tocopherol, ascorbic acid, and tartaric acid. The replacement of maleic acid with tartaric acid is driven by the proposed bio-friendly nature of the surfactant. Tartaric acid has inherent antioxidant properties and upon hydrolysis of the BFS in the body, it is hypothesized that three healthy components (tartaric acid, vitamin E and vitamin C), all with antioxidant properties, will result.
Characterization of the surfactant will be continued, and the applicability of the new BFS in nanoparticle synthesis will be proven during the second year of this grant. At least one publication will result from this research. A grant pre-proposal will be submitted in November of 2007 to the NSF Division of Chemistry: Collaborative Research in Chemistry Program.
Impact of PRF-G funding on the PI's professional development
The PI is indebted to Dr. Paul Russo, in the Chemistry Department at LSU for calling attention to the ACS-PRF, and to the PRF-G program for funding this proposal. Student stipends, supplies and chemicals were acquired with funds provided by this program. As a result of this program, a successful collaboration was initiated with Drs. Dolliver and Norwood, of the Chemistry-Physics Department at Southeastern Louisiana University. A cohesive team was created and chances for success for the PI and collaborators have increased significantly due to the funding obtained from the PRF-G program.