ACS PRF | ACS
All e-Annual Reports

43124-B3
Magnetic Studies and Room Temperature Carbon-Carbon Bond Cleavage in Ruthenium Carboxylate Clusters
Our investigations into the determination of a generalizable strategy for the synthesis and purification of oxo-bridged basic triruthenium carboxylate clusters have continued to establish that this deceptively simple system is actually exceedingly complex. As noted in our previous report, the common synthetic strategy:
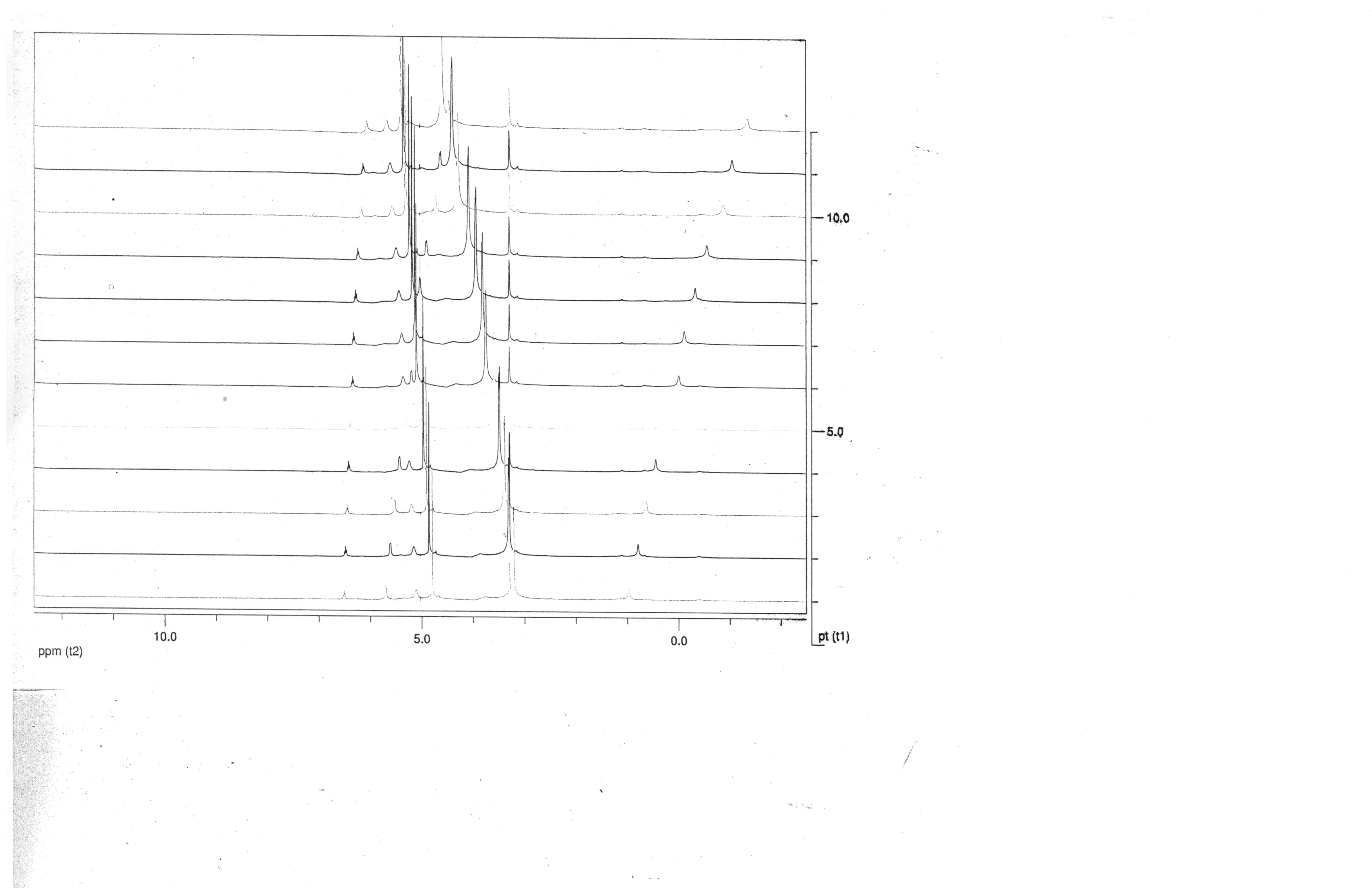
Figure 1. General strategy for the synthesis of basic ruthenium carboxylates is actually very sensitive to acid strength, even when carried out in organic solvents. Careful studies of the ubiquitous acetate system by Toma and co-workers1 have elucidated that the redox and acid-base transformations in the cluster can occur simultaneously in proton-coupled electron transfer (PCET) processes. These transformations, also seen in ruthenium polypyridyl systems studied by Meyer,2 feature conversion of aqua ligands to hydroxo and oxo ligands during simultaneous electron transfer. Prior to the publication of Toma's results, we elected to focus on finalizing the preparation and characterization of the [Ru3O(O2CCH(CH3)2)6(py)3]PF6 species.3 Unsurprisingly, the synthesis and isolation of the isobutyrate product displays the same PCET processes as the acetate species, thus making it critical to identify crucial details of the reaction to maximize the yield of the Ru3(III,III,III) species. Less than optimum reaction conditions lead to the isolation of green or black products which have yet to be definitively characterized, but 1H NMR has been used to distinguish them from the desired blue cationic species. Although large amounts of high-boiling carboxylic acids are difficult to remove from the initial reaction, they are required to produce the aqua species, [Ru3O(O2CCH(CH3)2)6(H2O)3]+ as the primary product. We are currently working to optimize the subsequent pyridine exchange step also to minimize the pH since the deprotonation of the aqua ligands to hydroxo groups blocks the axial ligand exchange. Our comprehensive characterization of the [Ru3O(O2CCH(CH3)2)6(py)3]PF6 species has been assisted by our collaborators, Prof. John Turner and Prof. Ted Barnes, both at the Figure 2. Stack plot of variable temperature 1H NMR spectra of [Ru3O(O2CCH(CH3)2)6(py)3]PF6 This grant award has had substantial impact on my student and I. At the References 1. Nunes, G. S.; Alexiou, A. D. P.; Araki, K.; Formiga, A. L. B.; Rocha, R. C.; Toma, H. E. Eur. J. Inorg. Chem. 2006, 1487-1495. 2. Meyer, T. J.; Huynh, M. H. V. Inorg. Chem. 2003, 8140. 3. Pink. C. C.; Saad, N. L.; Schlough, J. M.; Mugge, A. M.; Pence, L. E. manuscript in preparation.