
ACS PRF | ACS
All e-Annual Reports

44893-B7
Aggregation and Liquid Crystal Properties of Two Chromonic Systems
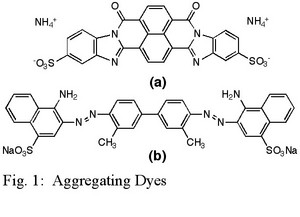
The goal of the grant was to investigate two new dyes that form chromonic liquid crystals, Bordeaux Ink and Benzopurpurin 4B (Figs. 1(a) and 1(b), respectively). The former has a structure quite different from the other dyes previously studied and the latter is reported to have a liquid crystal phase at very low concentrations.
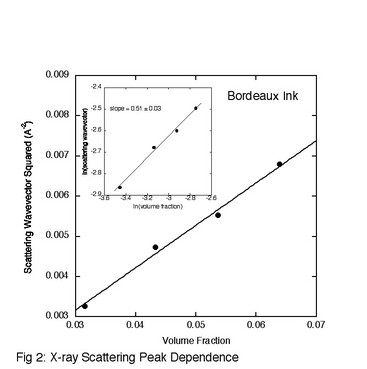
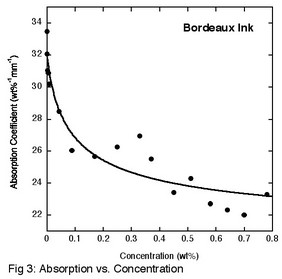
Bordeaux ink, which is the product of the sulfonation of the cis isomer of naphthalenetetracarboxylic acid, was investigated using optical retardation, x-ray scattering, and absorption spectroscopy. The optical retardation measurements mapped out the phase diagram, demonstrating that the liquid crystal phase formed at room temperature for concentrations above about 5 wt%. This is slightly less than for a frequently studied system and significantly less than a recently studied system. The small angle x-ray scattering investigation demonstrated that the aggregates are columnar (the slope of the inset in Fig. 2 is one-half) and that the cross-section of the columnar aggregates is equal to the cross-section of 2-3 molecules (calculated from the slope of Fig. 2). This contrasts to what occurs in a previously studied system where the cross-section is composed of a single molecule. Finally, the absorption coefficient was measured as a function of concentration. Using the exciton model for the interaction between molecules in an aggregate and a simple theory of isodesmic aggregation into columns, a fit to the absorption data reveals that the change in free energy per molecule when joining an aggregate is (15.3 ± 0.5) kT (Fig. 3). This result is higher than for the one other system studied, but must be true since Bordeaux Ink forms a liquid crystal phase at lower concentrations.
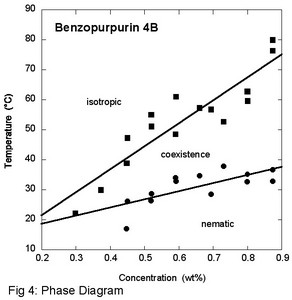
Benzopurpurin 4B has a very different structure from Bordeaux ink. It is a textile dye, and as a result contains a good deal of salt. Purification was achieved by dialysis, first against NaCl and then against water. The phase diagram of the pure compound demonstrates that the liquid crystal phase forms at extremely low concentrations, about 0.3 wt% (Fig. 4), which has been reported previously in the literature. X-ray scattering experiments show no peaks corresponding to aggregate - aggregate distances, so the aggregates must be much larger than in just about all the other chromonic liquid crystal systems studied. The aggregates cannot be too large, however, because light scattering measurements do not give evidence for aggregates larger than micron size. Absorption spectroscopy reveals an absorption coefficient that varies much like in other aggregating systems, but as one would expect from the low concentrations, the change in free energy per molecule upon aggregation is higher, namely (17.8 ± 0.3) kT (Fig. 5).
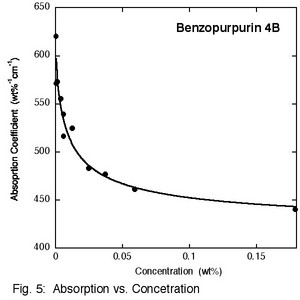
Future work will be directed at determining more about the structure of the aggregates of Benzopurpurin 4B. The only way a liquid crystal phase can form when the concentration is tenths of a percent by weight is for the aggregates to contain water. This might allow the volume fraction of the aggregates to be large enough to form an ordered phase even though the volume fraction of the dye is much smaller. Further light scattering studies will be performed, the effect of added salt will be investigated, and powerful microscopic techniques will be employed.