
ACS PRF | ACS
All e-Annual Reports

40944-B3
Investigation of the Reactivity of Imidoruthenium Complexes
Redox Transformations of [Ru(bpy)2(mpea)]2+ 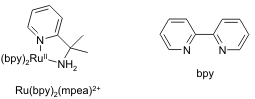 We have completed a detailed analysis of the redox chemistry of [Ru(bpy)2(mpea)]2+ (mpea = 1-methyl-1-(pyridin-2-yl)ethylamine; bpy = 2,2'-bipyridine). Of key interest are the interaction of the metal center with the amine site of the mpea ligand and the ability of the metal to catalyze oxidation of the amine. During October 2006, Davidson College acquired a new 32-processor computer cluster for computational chemistry. This cluster has permitted us to perform extensive density functional computations for the [Ru(bpy)2(mpea)]2+ system. These computations have been invaluable in interpreting the experimental data and understanding the factors controlling reactivity in this system.
We have completed a detailed analysis of the redox chemistry of [Ru(bpy)2(mpea)]2+ (mpea = 1-methyl-1-(pyridin-2-yl)ethylamine; bpy = 2,2'-bipyridine). Of key interest are the interaction of the metal center with the amine site of the mpea ligand and the ability of the metal to catalyze oxidation of the amine. During October 2006, Davidson College acquired a new 32-processor computer cluster for computational chemistry. This cluster has permitted us to perform extensive density functional computations for the [Ru(bpy)2(mpea)]2+ system. These computations have been invaluable in interpreting the experimental data and understanding the factors controlling reactivity in this system.
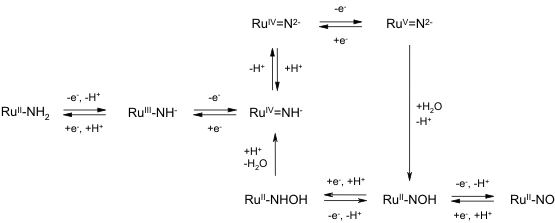 Oxidation of the complex occurs through a series of one-electron transformations leading to an imidoruthenium(V) intermediate, which is subject to attack by water, ultimately yielding a nitrosoruthenium(II) product. Reduction of the nitrosoruthenium(II) complex ultimates returns the structure to the original amineruthenium(II) complex, [Ru(bpy)2(mpea)]2+. The reactivity of this system is summarized in the following scheme. The density functional computations have provided key insights into the factors controlling attack by water on the amine nitrogen. Redox Transformations of poly-[Ru(AQ)3]2+
Oxidation of the complex occurs through a series of one-electron transformations leading to an imidoruthenium(V) intermediate, which is subject to attack by water, ultimately yielding a nitrosoruthenium(II) product. Reduction of the nitrosoruthenium(II) complex ultimates returns the structure to the original amineruthenium(II) complex, [Ru(bpy)2(mpea)]2+. The reactivity of this system is summarized in the following scheme. The density functional computations have provided key insights into the factors controlling attack by water on the amine nitrogen. Redox Transformations of poly-[Ru(AQ)3]2+
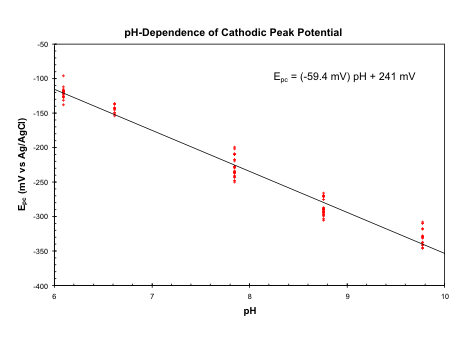 Oxidation of [Ru(AQ)3]2+ yields an electroactive polymer that displays a broad voltammetric wave. Coulometry indicates that 0.96 +/- 0.04 electrons are passed for each ruthenium in the polymer, suggesting a one-electron process in which essentially all of the ruthenium centers are electroactive. The anodic portion of the wave shows a large, sharp peak that is strongly dependent upon sweep rate, moving to increasingly negative potentials as the sweep is reduced down to 1 mV/sec. In contrast, the position of the cathodic peak is relatively independent of sweep rate below 20 mV/sec. The variation of the cathodic peak potential (Epc) with the solution pH for a single polymer film (in the presence of 1 M KCl) is shown below. (The reproducibility of the measurements may be judged by the scatter in the points.) The variation of Epc with pH is linear with a slope of -59 mV, indicating a one-electron, one-proton process. Our working hypothesis is that poly-[Ru(AQ)3]2+ is formed by coupling quinoline radicals when Ru(AQ)32+ is oxidized. Like the [Ru(bpy)2(mpea)]2+ system described above, the first step in oxidizing the poly-[Ru(AQ)3]2+ is expected to be a one-electron, one-proton oxidation of the amineruthenium(II) sites to amidoruthenium(III) species. The variation in the anodic peak potential with sweep rate is consistent with an EC mechanism. Following the initial oxidation of the polymer (presumably from RuII-NH2 to RuIII-NH-), the polymer apparently undergoes a structural or conformation change to a more stable state. The reduction of RuIII-NH- to RuII-NH2 sites presumably occurs at this new conformation, with only slow reversion to the original polymer state.
Oxidation of [Ru(AQ)3]2+ yields an electroactive polymer that displays a broad voltammetric wave. Coulometry indicates that 0.96 +/- 0.04 electrons are passed for each ruthenium in the polymer, suggesting a one-electron process in which essentially all of the ruthenium centers are electroactive. The anodic portion of the wave shows a large, sharp peak that is strongly dependent upon sweep rate, moving to increasingly negative potentials as the sweep is reduced down to 1 mV/sec. In contrast, the position of the cathodic peak is relatively independent of sweep rate below 20 mV/sec. The variation of the cathodic peak potential (Epc) with the solution pH for a single polymer film (in the presence of 1 M KCl) is shown below. (The reproducibility of the measurements may be judged by the scatter in the points.) The variation of Epc with pH is linear with a slope of -59 mV, indicating a one-electron, one-proton process. Our working hypothesis is that poly-[Ru(AQ)3]2+ is formed by coupling quinoline radicals when Ru(AQ)32+ is oxidized. Like the [Ru(bpy)2(mpea)]2+ system described above, the first step in oxidizing the poly-[Ru(AQ)3]2+ is expected to be a one-electron, one-proton oxidation of the amineruthenium(II) sites to amidoruthenium(III) species. The variation in the anodic peak potential with sweep rate is consistent with an EC mechanism. Following the initial oxidation of the polymer (presumably from RuII-NH2 to RuIII-NH-), the polymer apparently undergoes a structural or conformation change to a more stable state. The reduction of RuIII-NH- to RuII-NH2 sites presumably occurs at this new conformation, with only slow reversion to the original polymer state.