ACS PRF | ACS
All e-Annual Reports

43465-AC10,6
Surface Chemistry of III-V Compound Semiconductors with Novel Cluster Models
(1) Refinement and Assessment of New Cluster Models
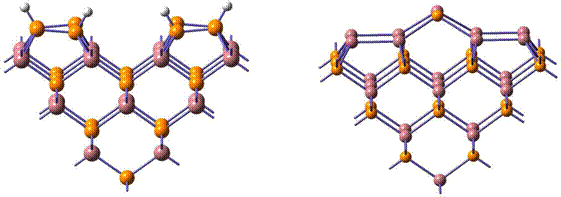
We have now refined our large cluster models to accurately describe the structures and chemical reactions of adsorbates on indium phosphide surfaces. In particular, our models include the proper balance between covalent and dative bonding present in compound semiconductors, and are large enough to incorporate neighboring dimer effects on the predicted reaction energies as well as vibrational frequencies for adsorbate species. Figure (1-left) is a schematic representation of a H-stabilized P-rich surface containing four buckled phosphorus dimers each with one adsorbed hydrogen. Figure (1-right) represents an In-rich InP(001)-d(2×4) surface containing four indium dimers and an indium-phosphorus mixed dimer.
(2) Mechanism of MetalOrganic Vapor Phase Epitaxy (MOVPE)
A clear understanding of the surface chemistry that controls the MOVPE growth of compound semiconductors is lacking. In our recent work, we have used density functional calculations using our cluster models to investigate (a) the growth of the In-rich indium phosphide surface resulting from the chemical reaction of InH3 with the H-stabilized P-rich (2×1) surface, and (b) the growth of the P-rich (2×1) surface resulting from the chemical reaction of In-rich InP(001)-(2×4) surfaces with PH3. Our studies provide the basic framework to understand the chemistry of compound semiconductors and yield new chemical insights into such growth processes.
(a) In-rich Surface Growth on P-rich InP(001) (2×1) Surface
A sequence of reactions involving physisorption of InH3, dissociative chemisorption involving hydrogen migration, and the formation of intradimer as well as interdimer P–In–P linkages accompanied by H2 elimination reactions leads from a P-rich surface to an In-rich surface. The initial barrier (~15 kcal/mol) for the InH3 dissociation on the phosphorous dimer is the rate-determining step. Interdimer P–In–P bridge formation in the intervening space between two phosphorous dimers is kinetically as well as thermodynamically more expensive compared to intradimer P–In–P bridge formation.
(b) PH3 adsorption on In-rich InP surfaces and Subsequent Chemical Reactions
Initially, the phosphine molecules form dative bonds with surface indium atoms. Subsequent dissociation leads to PH2 groups covalently attached to the surface along with a In–H–In bridged hydride containing a 3c–2e bond. Comparison between computed and experimental surface infrared frequencies clearly shows that both dative bonded PH3 and PH2 species are present at room temperature. In spite of its relatively low binding energy (~11 kcal/mol), the dative bonded state is found mainly because of a very high energy barrier (~21 kcal/mol) associated with PH3 dissociation.
We have also identified the key reactions at high temperature that lead to the formation of P–P bonds characteristic of a P-rich surface. The primary products of these reactions are the bridged phosphides formed by the insertion of PH2 and PH groups into the surface In–In dimers. We propose an alternating growth of bridged PH2 and PH along the [110] axis followed by H2 elimination reactions. Such a sequence of reactions maintains the proper surface electron distribution. Interestingly, the rate determining step for all these surface chemical reactions is the initial phosphine dissociation. At higher temperatures, the P–P dimer bonds break and desorb P2 molecules to regenerate the In-rich reconstruction. This process is endothermic.
(3) Compound Semiconductor Alloys: Ordering in Growth of InGaP2(001) Surface
In this work, the thermodynamics and kinetics of the key steps involved in the growth mechanism of the Group III (Ga or In) sublattice over P-rich surface to yield CuPtB ordering have been investigated. It is clearly demonstrated using cluster model calculations that the ordering process is purely a kinetically driven surface phenomenon.
(4) Surface Passivation of Indium Phosphide
We are currently investigating the passivation of In-rich indium phosphide surfaces with many species such as H2O, CH3OH, and H2S. For example, in the reactions of H2S with the surface, the formation of physisorbed molecules as well as chemisorbed species involving thiol linkages have been found. In addition, passivated surface-inserted species containing In–S–In linkages are formed via hydrogen migration and H2 desorption.
(5) Bond length–frequency correlations
During the course of this study, we have computed a large number of surface PH vibrations with different bonding environments such as dative bonded PH3, covalently attached PH2, bridged PH2 and PH, etc. It is well known that the isolated frequencies of such high-frequency modes correlate linearly with the corresponding bond lengths for small gas phase molecules. In our work, we have observed a similar relationship for the surface vibrations with a small dependence on the local coordination. By a careful analysis, we can now use such correlations to predict the isolated vibrational frequencies without computing the force constant matrix explicitly.