ACS PRF | ACS
All e-Annual Reports

45004-B10
Investigating the Chemistry of Boranes with Single-Walled Carbon Nanotubes Using FTIR and Raman Spectroscopies
We have made some progress in our research toward reacting boron-containing compounds with single-walled carbon nanotubes (SWCNTs). I took very seriously the warning of one reviewer who said, “…my recommendation would be to drop [the reaction of diborane with SWCNTs] in favor of an expanded effort on the liquid phase studies that use BH3 complexes.” Our work thus far indicates that the BH3 complexes we have tried, BH3-THF and BH3-triethylamine, do not react with SWCNTs. For BH3-THF and SWCNTs at 0°C or 25°C, infrared and Raman spectroscopies indicate that no reaction takes place. For BH3-triethylamine and SWCNTs at 0°C, 25°C, or 65°C, spectroscopic analysis also indicates no reaction. Presumably, under these conditions, the borane complexes are not reactive enough to attack the conjugated pi system of the SWCNTs.
Consequently, we determined to try a more reactive boron-containing compound, diborane. One way to generate small quantities of diborane gas is the reaction of phosphoric acid with sodium borohydride:
2H3PO4(l) + 6NaBH4(s) à 3B2H6(g) + 3H2(g) + 2Na3PO4(s)
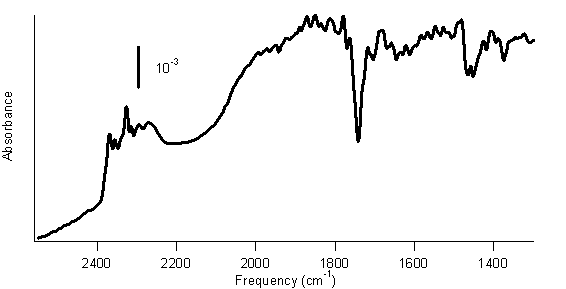
Because phosphoric acid is a non-oxidative acid, we expect that it will not react with SWCNTs, but we will of course run control experiments. Figure 1 shows a preliminary FTIR spectrum of SWCNTs reacted with diborane gas produced using the above reaction.
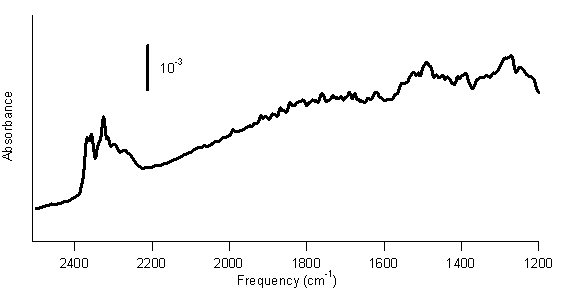
Figure 1. FTIR spectrum of SWCNTs reacted with diborane gas. In this spectrum, peaks at 2200–2400 cm–1 are present. These peaks are consistent with the B–H stretching vibrations of a –BH2 group. Thus, this spectrum suggests that the SWCNTs have been functionalized with –BH2 groups. Therefore, we will continue to study this reaction. We also have preliminary results suggesting that a photochemical reaction between BH3-THF and SWCNTs is possible. SWCNTs were immersed in BH3-THF under a nitrogen atmosphere and subjected to radiation from a mercury lamp for 2 h. Figure 2 shows the FTIR spectrum of the product of this process. Figure 2. FTIR spectrum of SWCNTs photochemically reacted with BH3-THF. Figure 2 shows peaks from 2200–2400 cm–1, again consistent with B–H stretches. In this spectrum, peaks at 1490 cm–1 and 1275 cm–1 are apparent. Previous researchers have associated these peaks with boron-substituted SWCNTs.1 Consequently, these data indicate that the SWCNTs might be functionalized with –BH2 groups. Therefore, we will pursue the photochemical reaction of BH3-THF with SWCNTs. We have had some other successes in the research. Previously developed methods of functionalizing SWCNTs include attaching a benzene ring with an –NO2 group para to the bond to the SWCNT. Later reserachers used this diazonium reaction, followed by an electrochemical reduction of the –NO2 group to an –NH2 group. Our work demonstrates that this reduction can be achieved using the BH3-THF or BH3-triethylamine complex, which is simpler and avoids most of the complications of the electrochemical reduction. Another side project that is ongoing is the use of borane complexes to reduce oxygen-containing functional groups on SWCNTs. Purified SWCNTs contain functional groups such as carboxylic acids, ketones, and aldehydes.2,3 Borane complexes can reduce these functional groups, so we are purifying SWCNTs with strongly oxidizing acids, such as nitric acid, to produce SWCNTs with significant numbers of oxygen-containing functional groups. We will then attempt to reduce these functional groups with BH3-THF or BH3-triethylamine. This grant has been acknowledged in one submitted article, reference 4. A research student designed and carried out a project to attach DNA to SWCNTs. His work was not directly supported by this ACS PRF grant. However, he did use SWCNTs purchased with funds from this grant. Therefore, I thought it appropriate to acknowledge this ACS PRF grant in the manuscript. A total of 7 students have worked or are working on the experiments described above. Five of these students presented posters on their research at the Mid-Atlantic Regional Meeting of the ACS on May 16, 2007. References (1) Borowiak-Palen, E., Pichler, T.; Fuentes, G. G.; Graff, A.; Kalenczuk, R. J.; Knupfer, M.; Fink, J. Chem. Phys. Lett. 2003, 378, 516. (2) Feng, X.; Matranga, C.; Vidic, R.; Borguet, E. J. Phys. Chem. B 2004, 108, 19949. (3) Kuznetsova, A.; Popova, I.; Yates, J. T., Jr.; Bronikowski, M. J.; Huffman, C. B.; Liu, J.; Smalley, R. E.; Hwu, H. H.; Chen, J. G. J. Am. Chem. Soc. 2001, 123, 10699. (4) Ellison, M. D.; Gasda, P. J. submitted to Journal of Physical Chemistry C 2007.