ACS PRF | ACS
All e-Annual Reports

44165-G5
Synthesis and Kinetic in situ Structural Investigations of Pt Alloy Nanoparticle Catalysts
The performance of hydrogen fuel cells is limited by the rate of reduction of molecular oxygen on Pt nanoparticle catalysts. Pt alloys have shown improved activity, yet their structural and compositional stability in the corrosive environment of a fuel cell cathode is poorly understood.
This work aims to in-situ characterize the dynamics of particle size and particle composition distributions of Pt alloy fuel cell nanoparticle catalysts under corrosive electrochemical conditions. In-situ Small-Angle X-ray Scattering (SAXS) and X-ray diffraction (XRD) (see Figure 1) are utilized to measure particle size and composition distributions under reactive conditions. Also, the nature of the alloy phases as a function of synthesis parameters and electrocatalytic reaction conditions is explored. While in-situ SAXS focused on the change in particle size distribution, in-situ XRD probes the cell parameters of the Pt alloys as function of time and applied potential. Both methods combined provide complementary insight in the structural and compositional stability of alloy particle electrocatalyst ensembles. The goal of this work is to derive synthesis-structure-stability relationships of Pt alloy nanoparticle ensembles. Such relations provide guidance for developing synthetic strategies for highly monodisperse alloy particle ensembles and, in addition, yield kinetic information on the rate of change in particle size and composition due to electrochemical de-alloying processes.
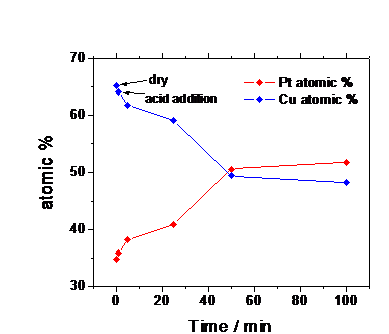
Fig 1: Principle of in-situ real-time probing of structural transformations of nanoparticle electrocatalysts due to metal leaching under electrified conditions. We have designed an in-situ electrochemical flow XRD and SAXS chamber in which alloy particle electrocatalysts will be supported on a carbon paper working electrode (WE). Liquid electrolyte, counter and reference electrode complete the electrochemical environment (Fig.2). We have developed a new class of improved Pt-Cu alloy nanoparticle electrocatalysts for the electroreduction of oxygen at fuel cell cathodes. The catalysts catalyzed the reaction about 4-6 times more efficient than conventional pure Pt electrocatalysts. Catalyst pretreatment during which electrochemical dissolution of Cu atoms fomr the precursor alloy occurs turned out to be crucial for the preparation of the active phase of the catalyst. We have performed in situ X-ray diffraction (in situ XRD) studies of Pt-Cu precursor catalysts in order to study the kinetics of the structural changes that occur during the voltammetric preparation of the active catalyst phase. Figure 3 shows the diffraction profiles of a Pt25Cu75 precursor catalyst as function of time over a period of 100 minutes. The catalyst is electrochemically dealloyed at a constant potential of about 1.6 V versus Ag/AgCl. The measurement indicates a substantial shift of the fundamental (111) reflection suggesting a gradual expansion of the unit cell parameters due to the continuous preferential dissolution of Cu from the Cu rich precursor. Detailed compositional analysis of the catalyst as a function of time is presented in Figure 4. Our measurements evidence that the active phase of the oxygen reduction electrocatalyst consists of a strongly Pt enriched alloy phase. We have also conducted ASAXS measurements of Cu rich Pt-Cu alloy electrocatalysts precursors and have established synthesis-structure relationships between annealing temperature on the particle size distribution of the resulting alloy material. Higher annealing temperature result in particle distributions shifted toward larger particles. . Fig. 2: Electrochemical-SAXS(XRD) cell for in situ investigations of porous fuel cell electrodes. (a) Schematic illustration. REF = reference electrode, WE = working electrode and CE = counter electrode. (b) Photograph of prototype cell. A very complex particle size dynamics was observed during the initial voltammetric dealloying of Cu rich catalyst precusors using in situ SAXS. Detailed nonlinear fitting of the various Intensity-Scattering Vector profiles revealed that, initially, the catalyst particles reduce their mean particle size due to the preferential dissolution of Cu atoms, however, at later stages of the electrochemical treatment, the particle size increases due to the electrochemical treatment. In conclusion, we have successfully developed and utilized in-situ synchrotron-based X-ray scattering capabilities of Pt-Cu nanoparticle electrocatalysts for the electroreduction of oxygen. Our studies reveal the complex dynamics of particle size and composition as function of synthesis conditions and electrochemical treatment. Fig. 3: In situ X-ray diffraction profile of a Pt35Cu65 nanoparticle electrocatalystover 100 min during electrochemical leaching. Fig. 4: Composition time profile measurement obtained from in situ X-ray diffraction studies in Fig. 3.