ACS PRF | ACS
All e-Annual Reports

40318-B1
The Furan Approach to the A-Ring of Vitamin D Analogues
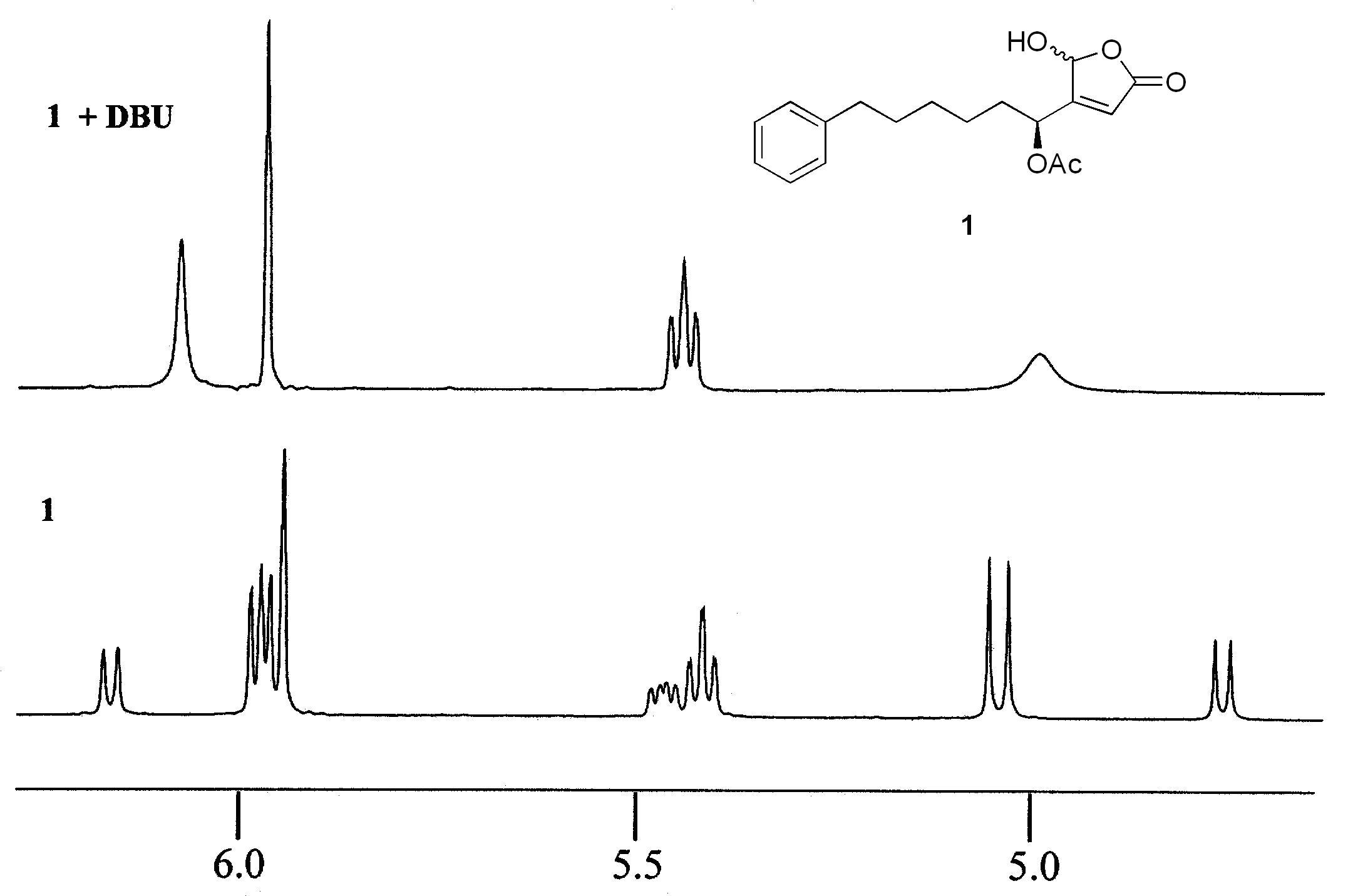
In the course of preparing and studying chiral γ-hydroxybutenolides, we encountered an issue commonly recognized in the field, the epimerization of the C-5 chiral center. For most of the γ-hydroxybutenolides we investigated, the NMR spectra had two well-defined sets of peaks assignable to the two epimers, indicating slow exchange on the NMR timescale. In an attempt to simplify the NMR spectra, we looked at various amines as additives, with the initial hope of forming the conjugate base, which exists as the open form carboxylate. In our studies, however, we uncovered the remarkable ability of amines to catalyze the epimerization of chiral γ-hydroxybutenolides. The partial 1H NMR for the DBU-catalyzed epimerization of 1, for example, is given below. Our study calls into question a recent report that claims to have prepared a series of epimerically pure γ-hydroxybutenolides.