ACS PRF | ACS
All e-Annual Reports

43626-AC6
Measurements of Structures for Organometallic Complexes Using FT Microwave Spectroscopy
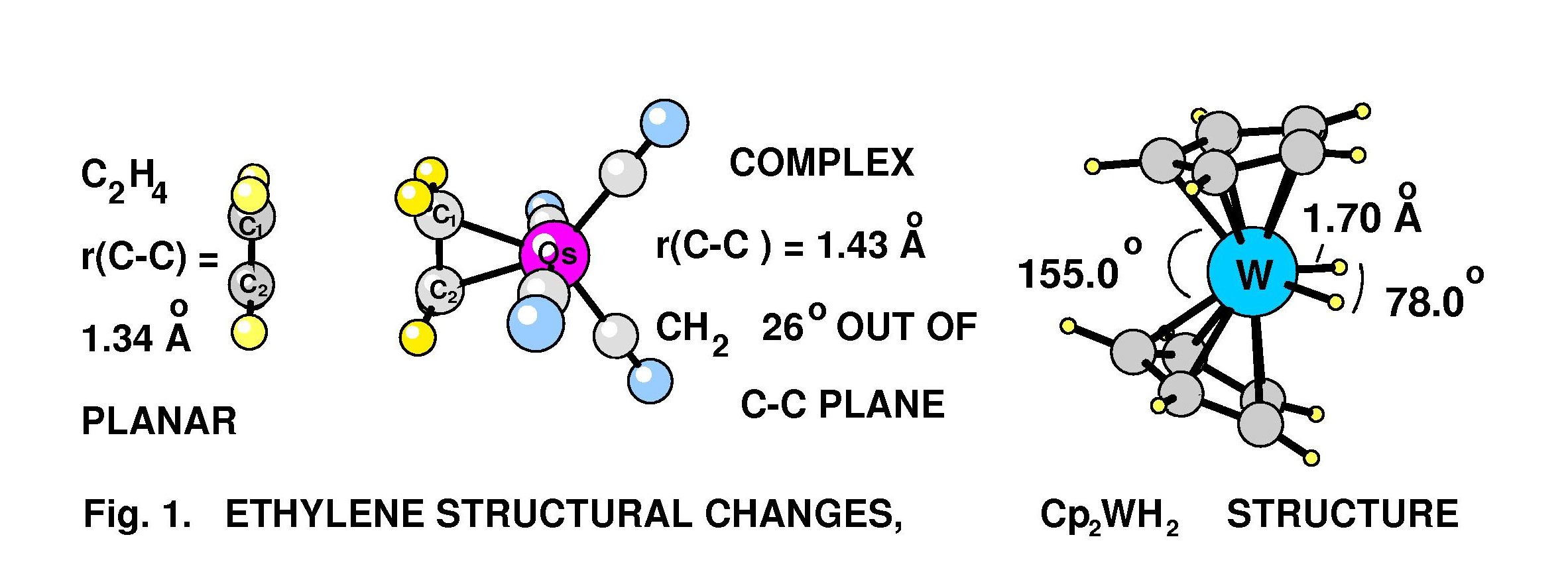
Transition metal complexes are becoming very widely used in organic syntheses and production of chemical products from petroleum derivatives. The understanding of reaction mechanisms, which is often quite useful in the development of more efficient catalysts, can be aided by accurate structural information on transition metal complexes. Microwave spectroscopy provides accurate and precise, gas-phase structural information on transition metal complexes.This PRF grant supported microwave structural studies on tetracarbonylethyleneosmium and cyclopentadienyltungsten dihydride cpmplexes, and high-resolution IR measurements on cyclopentadienyl nickel nitrosyl. A near-complete r0 gas-phase structure has been determined for Os(CO)4(h2-C2H4) from the measured rotational spectra of seven isotopomers. Accurate and precise ethylene carbon and hydrogen atom coordinates were obtained. Olefin-transition metal complexes of this type serve as important models of transition states in metal-mediated transformations of alkenes. Changes in the structure of ethylene on coordination to Os(CO)4 are large and well-determined. For the complex the experimental ethylene C—C bond length is 1.432(5) Å, which falls between the free ethylene value of 1.3391(13) Å and the ethane value of 1.534(2) Å. The angle between the plane of the CH2 group and the extended ethylene C—C bond (angle out-of-plane) is 26.0(3)°, indicating that this complex is better described as a metallacyclopropane than a p-bonded olefin-metal complex (see Fig. 1). The formation of transition metal complexes with alkenes and many other organic molecules causes large and significant changes in the structure and reactivity of the organic ligand. It is well recognized that the details of the metal-alkene bond have a profound influence on the catalytic activity of the metal complex, and the reactivity of the coordinated alkene. Ethylene structural changes upon coordination to the metal are found to be larger for the ethylene-osmium complex than for the analogous ethylene-iron complex studied earlier in our lab. The first microwave study of a bent metallocene complex, bis(h5-cyclopentadienyl)tungsten dihydride ((C5H5)2WH2), was recently published. Microwave spectra for 11 isotopomers of this complex were obtained and analysed to obtain to determine the W—H bond length, r(W-H) = 1.703(2) Å, the H-W-H bond angle = 78.0(12)°, the W-Cp centroid distance, r(W-Cp) = 1.940(8) Å, and other structural parameters. The results indicate that this is clearly a “classical dihydride” rather than an “h2-dihydrogen” complex. A homodyne-type detection system was developed for our pulsed-beam Fourier transform spectrometer system and used for these measurements. This homodyne system greatly simplifies the microwave circuit, with no apparent loss in sensitivity. Gas-phase rotational constants and distortion constants have been determined for an excited vibrational state of cyclopentadienylnickel nitrosyl (C5H5NiNO) using a high resolution Fourier transform spectrometer (FTS).The rotationally resolved lines have been measured for the C-H symmetric stretch vibration (n 1=3110 cm-1).In the present analysis, one hundred fifty five lines have been assigned and fitted to obtain the vibrational band center, excited state rotational constants and distortion constants. Values are n 1 = 3110.4129(4) cm-1, A' = 0.14328(8) cm-1, B' = C' = 0.041285(1) cm-1, DJ' =0.078(1) kHz, DJK'= 2.23(4) kHz and DK'= -2.63(2) kHz.Several different lower state combinations differences were calculated for different K – stacks for the observed spectra and provided support for the assignments.The C-H stretch vibrational frequency and rotational constants were calculated using Density Functional Theory (DFT) calculations and these were quite helpful in resolving ambiguities in fitting procedure and for initial assignments of measured lines.