
ACS PRF | ACS
All e-Annual Reports

44139-GB3
Doubly Bridged Metallocenes with Two Distinct Interannular Linkers: Synthesis and Regioselectivity for alpha-Olefin Polymerization
During the past year, my research group has worked towards the synthesis of two families of zirconium compounds with cyclopentadienyl-based ligands. These targets are: (1) doubly bridged zirconocenes with two distinct interannular linkers, and (2) singly bridged zirconocenes with one bulky bridging group, designed to favor meso-metallation. The scheme below illustrates our progress to date. 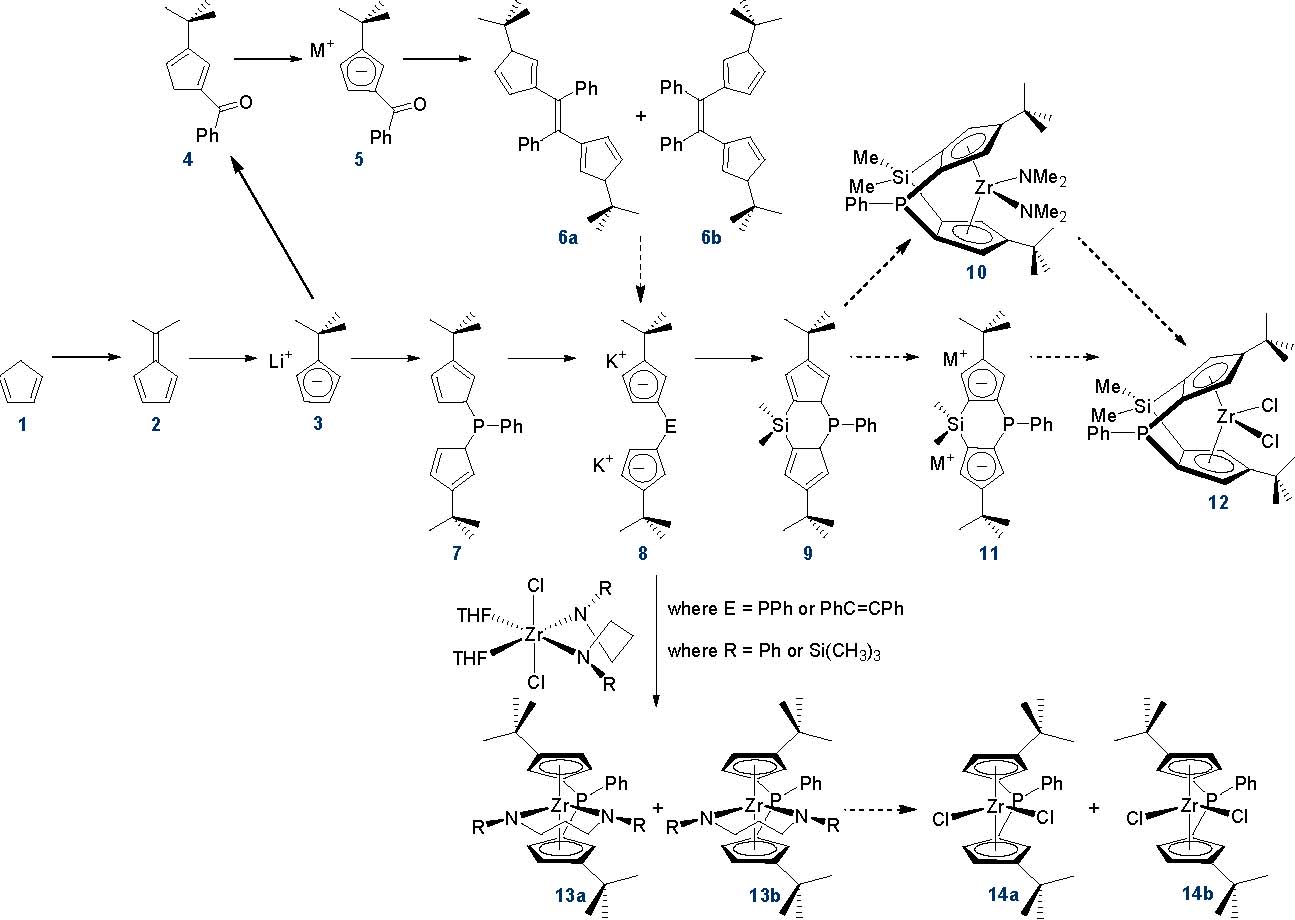
The synthesis of one doubly bridged target, [(Me2Si)(PhC=CPh){C5H2-4-t-Bu}2]ZrCl2 was initiated prior to the grant period and has continued during the grant period. Installation of the alkene linker has proven to be particularly challenging. Addition of distilled methyl benzoate to 3 affords 4 in 65% yield after flash silica gel chromatography as a mixture of two isomers (one isomer is shown). Deprotonation of 4 using KN(SiMe3)2 provides air-sensitive 5 in 74% yield. McMurry coupling of 4 (to form 6a/6b) or 5 (to form 8, cis and trans) was attempted using two different sets of coupling agents for multiple reaction trials. Coupling agents KC8/TiCl3(THF)3 (in THF solvent) and Zn-Cu/TiCl3(DME)1.5 (in DME solvent) were tested. One reaction trial using KC8/TiCl3(THF)3 seemed promising based on a crude 1H NMR spectrum and GC-MS analysis; the desired mass for 6a/6b was detected. However, this result was not reproducible. Purification was hindered by the presence of spent coupling reagent. Indeed, one reaction trial using Zn-Cu/TiCl3(DME)1.5 provided temperature-sensitive, X-ray quality crystals of TiCl2(THF)2. This project has been suspended temporarily.
Greater progress has been made in the synthesis of a second doubly bridged target, [(Me2Si)(PhP){C5H2-4-t-Bu}2]ZrCl2 (12). Compound 7 has been prepared in 85% yield as a mixture of double bond isomers; it is formed by the addition of chlorodiphenylphosphine to a tetrahydrofuran/pentane solution of two equivalents of 3. Double deprotonation of 7 with potassium t-butoxide in diethyl ether solution and subsequent recrystallization affords 8 as a white powder in 94% yield. The 31P NMR resonance at -31.14 (in THF-d8) for 8 is typical. With 8 in hand, we envision further functionalization to provide doubly bridged 12. Addition of a dimethylsilyl linker to 8 has been attempted by combination with dichlorodimethylsilane; purification of the reaction mixture is underway.
Using an intermediate for the synthesis of 12, preparation of singly bridged zirconocenes with one bulky phenylphosphine linker has also been achieved. We became interested in using some of Jordan's chemistry (JACS 2007, 129, 3468-3469) to evaluate the effect of our phenylphosphine linker on rac/meso metallation preferences. Specifically, we have used literature procedures to prepare both Zr[PhN(CH2)3NPh](THF)2Cl2 and Zr[Me3SiN(CH2)3NSiMe3](THF)2Cl2 and then used these reagents to synthesize singly bridged metallocenes of the form rac/meso-{(PhP)(C5H3-3-t-Bu)2}Zr{RN(CH2)3NR} (where R is phenyl or trimethylsilyl, 13a/13b). When Zr[PhN(CH2)3NPh](THF)2Cl2 is used for metallation of 8 using diethyl ether solvent, a mixture of rac and meso products is formed, with meso as the predominant product. NMR spectroscopy (1H, 13C, 31P, COSY, HETCOR, and 31P decoupled 1H NMR) has been used for structural assignments. Since these zirconocenes were isolated as an orange microcrystalline solid, we are hopeful that we may be able to obtain x-ray quality crystals in the near future. In a similar vein, when Zr[Me3SiN(CH2)3NSiMe3](THF)2Cl2 is used for metallation of 8 using tetrahydrofuran solvent, a mixture of rac and meso products is formed, with rac as the predominant product. Again, NMR spectroscopy (1H, 13C, 31P, COSY, HETCOR, and 31P decoupled 1H NMR) has been used for structural assignments. These rac/meso preferences are consistent with Jordan's reports based on choice of zirconium reagent and solvent. An initial attempt to convert 13a/13b (where R = Ph) to 14a/14b using 2 equivalents of HCl showed promise, and warrants further examination.
Lastly, a new project was initiated to prepare a novel pyridine bis(imine) ligand with strategic placement of steric bulk. Like the bis-cyclopentadienyl ligands described above, this ligand is designed to address regioselectivity preferences for alpha-olefin polymerization. This project will be described further in the progress report for year two.
Five poster presentations (two at an ACS National meeting, three internal) and one oral presentation (at an ACS Regional meeting) were made to disseminate the work carried out during the grant period.