ACS PRF | ACS
All e-Annual Reports

43100-AC5
Fast X-ray Photoelectron Spectroscopy of Pd-Catalysed Cross-Coupling Surface Reactions
One of the most important processes in modern chemistry is the carbon-carbon bond-forming reaction, since it represents key steps in the construction of complex molecules from simple precursors. However not until the discovery and development in the 1970s of metal-catalysed cross-coupling reactions was there a simple and direct method for carbon-carbon bond formation between sp and sp3 centres. A wide variety of metal-catalysed cross-coupling reactions now exist and have become powerful tools, finding application in the synthesis of biological molecules, liquid crystals, macromolecules and also in supramolecular chemistry.
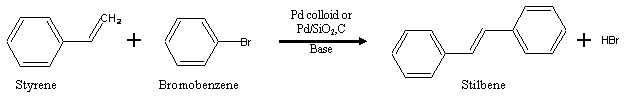
Pd-catalyzed cross-coupling reactions, such as Heck and Suzuki-Miyaura reactions, are used extensively in synthetic chemistry, and characterised by the coupling of an electrophilic species, such as organic halides, with a nucleophile, such as an alkene or organoboron, catalysed by Pd0. This catalysis is not confined to organometallic Pd complexes in the homogeneous phase; palladium colloids and supported Pd particles show good activity in Heck coupling (Scheme 1) and this proposal addresses the underlying fundamental surface chemistry.
|
Scheme 1: Heterogeneously catalysed Heck coupling reaction
|
Over the past year Dr. Zhipeng Chang, the post-doctoral fellow appointed to this grant, has continued to explore the surface chemistry of several aryl halides, allylic alcohols and aldehydes pertinent to Heck coupling reactions, over catalytically relevant Pd and Pt model surfaces. These studies have involved synchrotron Fast XPS and NEXAFS measurements at both Elettra (
The surface chemistry of bromobenzene, one of the simplest activated aryl halides used in C-C cross-couplings, has been investigated over both Pd(111) and Pt(111) surfaces, alongside the unsubstituted benzene parent molecule. Time-resolved XPS shows that benzene adopts a single chemically distinguishable environment over Pt(111) within the monolayer, with a saturation coverage of 0.2 ML. This contrasts with Pd(111) wherein discrete tilted and flat-lying chemisorbed benzene form. Around 20% of a benzene monolayer desorbs molecularly over both surfaces, with the remainder dehydrogenating to surface carbon. Bromobenzene likewise adsorbs molecularly at 90 K over palladium and platinum, giving rise to two C 1s environments corresponding to the C-H and C-Br function. The saturation C6H5Br monolayer coverage is ~0.11 ML in both cases. NEXAFS reveals bromobenzene adopts a tilted geometry, with the ring plane around 60 ° to the surface. Bromobenzene multilayers desorb at 180 K, with higher temperatures promoting competitive molecular desorption versus C-Br scission within the monolayer. Approximately 30% of a saturated bromobenzene monolayer either desorbs reversibly or as reactively formed hydrocarbons. Debromination yields a stable (phenyl) surface intermediate and atomic bromine at 300 K. Further heating results in desorption of reactively formed H2, C6H6, and HBr. Although no coupling products desorbed from Pt(111), biphenyl production was observed over Pd(111) around 500 K. Both surfaces are efficient for low-temperature bromobenzene hydrodebromination to benzene and HBr, and palladium also proved capable of homo-coupling the associated phenyl surface intermediates. This latter exciting discovery provides the first unequivocal evidence that metallic Pd surfaces are able to perform the crucial oxidative addition and reductive elimination steps in the C-C bond-forming catalytic cycle. The bromobenzene/Pt(111) work has been published in Journal of Physical Chemistry C, and that on Pd is in preparation for the same journal.
|
Figure 1: Temperature-programmed reaction of a saturated bromobenzene adlayer over Pd(111) followed by C 1s Fast XPS. |
SHAPE \* MERGEFORMAT
 | ||||
Figure 2: Temperature-programmed C 1s Fast XP spectra of reacting crotyl alcohol saturation adlayer adsorbed at 100 K over Pd(111). |