
ACS PRF | ACS
All e-Annual Reports

44953-G5
Direct Measurement of Casimir Force in Critical Films of Binary Liquid Mixtures
The structure of liquids adjacent to a surface or macromolecule can be significantly different compared to the bulk. If the bodies are immersed in a fluid environment, which usually consists of multiple components, the local structure and composition of the fluids becomes modified again. One of the fluid components may preferentially adsorb onto the bodies because of the difference of their affinity for the solvent components. The width of this preferential adsorption layer is of the order of the solvent correlation length, which is typically a few angstroms, affecting only one or a few layers of liquid molecules next to the bodies. However, near the critical point of the liquid mixture the enhancement of adsorption of the preferred component becomes particularly pronounced due to the correlation effects induced by the critical composition fluctuations of the solvent. If two surfaces are brought within a distance of order of correlation length, then the interference of the composition profile can induce long-ranged “critical Casimir force” between them. For macromolecules, a preferential wetting layer can contribute to an indirect attraction among distant monomers within the polymer chain, resulting in dramatic changes to chain conformational behavior on approaching the critical point. Quantitative understanding of these and related phenomena has been impeded by the paucity of methods suited for their direct investigation. The support from ACS-PRF has allowed the PI to address some of these issues, which resulted in a publication at Physical Review Letters earlier this year.
Twenty-five years ago, Brochard and de Gennes made an interesting prediction regarding the conformational behavior of a flexible linear polymer chain in a critical binary liquid mixture. The better solvent, which is preferentially adsorbed onto the polymer, creates a cloud of good solvent around itself. This cloud attracts other segments of the polymer, thereby constituting an indirect long-range attraction among the distant monomers within the chain. The range of this interaction, driven by composition fluctuations, is the solvent correlation length. Since the correlation length diverges at the critical point, they reasoned that the polymer molecule should shrink and collapse when approaching the critical temperature (Tc). At temperatures sufficiently close to Tc, however, when the correlation length greatly exceeds the radius of gyration (Rg), the polymer coil will be completely immersed in the better solvent, causing the chain to re-expand. Their scaling arguments had later been confirmed by various theoretical calculations and simulations. On the experimental side, however, only the contraction of the polymer chain has been observed. By using a novel single-molecule sensitive fluorescence correlation spectroscopy (FCS) technique, we have studied the conformation change of a flexible linear polymer chain near the critical point of a binary liquid mixture. Our results indicate that as the Tc is approached, the chain-size decreases. The polymer attains its most compact conformation when the correlation length of the critical fluctuations becomes comparable to the coil size. At very close to Tc, the polymer re-expands dramatically. To our knowledge, this is the first experimental evidence supporting the prediction of Brochard and de Gennes that a polymer chain will collapse and subsequently re-swell on approaching Tc.
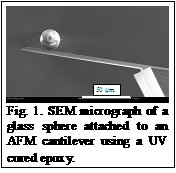 We have also made significant progress in measuring the critical Casimir force of a binary liquid mixture. We are using a home-built atomic force microscope (AFM) for this purpose. Previously, we faced significant design challenge in combining a surface forces apparatus (SFA) with a temperature controller system to achieve the required temperature stability. However, by using a commercially available proportional-integral-derivative (PID) temperature controller (Lakeshore Inc.) we have been successful in obtaining good temperature stability when using AFM. We have also succeeded in attaching a glass micro-sphere at the end of the AFM cantilever (Fig. 1). This is needed to obtain the well-defined parallel plate geometry and will allow us to compare the measured Casimir force with the theoretical predictions. One of the problems in all AFM experiments is the difficulty of determining the absolute distance between surfaces. We are implementing a new design, where by using two interferometers we can determine and control absolute distance at better than 0.1 nm scale.
We have also made significant progress in measuring the critical Casimir force of a binary liquid mixture. We are using a home-built atomic force microscope (AFM) for this purpose. Previously, we faced significant design challenge in combining a surface forces apparatus (SFA) with a temperature controller system to achieve the required temperature stability. However, by using a commercially available proportional-integral-derivative (PID) temperature controller (Lakeshore Inc.) we have been successful in obtaining good temperature stability when using AFM. We have also succeeded in attaching a glass micro-sphere at the end of the AFM cantilever (Fig. 1). This is needed to obtain the well-defined parallel plate geometry and will allow us to compare the measured Casimir force with the theoretical predictions. One of the problems in all AFM experiments is the difficulty of determining the absolute distance between surfaces. We are implementing a new design, where by using two interferometers we can determine and control absolute distance at better than 0.1 nm scale.