ACS PRF | ACS
All e-Annual Reports

42307-AC3
Hydrophilic and Lipophilic Systems for Catalytic Alkane Functionalization
Several directions aimed at stoichiometric and catalytic hydrocarbon functionalization were pursued:
1) aerobic functionalization of organoplatinum(II) complexes in water. This work led to a Journal of the American Chemical Society communication with an undergraduate co-author (Seth Binfield) and two Organometallics full papers (Julia Khusnutdinova). Two more papers, both with undergraduate student co-author (Laura Newman) are under preparation now;
2) stoichiometric and catalytic Pt-mediated functionalization of arenes, linear and cyclic alkanes in hydrocarbons and biphasic hydrocarbon/water media. This work resulted in two communications, one in the Journal of the American Chemical Society and another in Angewandte Chemie (Eugene Khaskin). Two full papers with undergraduate co-authors (Daniel Lew, Nicholas Anderson) are under preparation now;
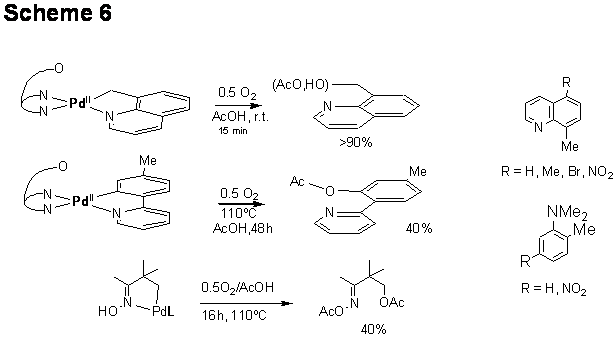
3) stoichiometric and catalytic donor-atom-directed palladacycles-mediated aerobic CH functionalization of organic amines in acetic acid. That was led by postdoc Jing Zhang and will be submitted as a JACS communication with an undergraduate student co-author (Nick Anderson); another manuscript is under preparation now.
During the grant period Julia and Eugene passed successfully their PhD candidacy examinations (Fall 2006). This PRF grant was the major source of financial support for them and Guoyuan Liu who received his M.S. degree in 2006. The grant support was tremendously important for the PI in the beginning of his carrier in 2. In our DFT-guided searches for water-tolerant systems for alkane CH activation, we discovered that anionic dimethyldi(4-R-2-pyridyl)borato dimethylplatinum(II) complexes react vigorously in biphasic RH/water mixtures to produce PtIIR2 (RH=C6H6, para-F2C6H4) or PtII hydrido olefin complexes (RH=n-C5H12, cyclo-CnH2n; n=5,6; Scheme 3). The presence of R=t-Bu is crucial for high yielding alkane dehydrogenation (>90% isolated yield; quantitative by NMR). Interestingly, the Lewis acidic Na+ cation accelerated the reaction rates dramatically. 3. One of the possible ways to do CH functionalization in hydroxylic solvents is with PdII analogues of the PtII complexes above. CH activation with palladium(II) acetate can be facile in acetic acid if it leads to palladacycles. We discovered that dpms and some other ligands promote aerobic oxidation of various cyclopalladated organic amines in acetic acid solution. The oxidation reactions are clean (Scheme 6) and some allow for catalytic aerobic oxidation of the amines to corresponding acetates (5%Pd(OAc)2, 80oC, 24h; 70-80%). Optimization and mechanistic studies might lead to more general application of this reaction and allow for aerobic oxidation of certain hydrocarbons including benzene and methane.  1. Unlike previously known monohydrocarbyl PtII complexes, (dpms)PtIIR(HX) (dpms = di-2-pyridylmethanesulfonate; HX=H2O, MeOH, EtOH; R=Me, HOC2H4 etc) can be readily oxidized by O2 at room temperature to produce (dpms)PtIVR(X)OH in quantitative yield (Scheme 1). A number of olefin complexes (dpms)PtII(olefin)OH undergo facile aerobic oxidation in water to produce PtIV alkyls (olefin=C2H4, cyclooctene, norbornene). We found that anionic intermediates (dpms)PtIIR(X)- are the key species responsible for the reaction with O2. The presence of the sulfonate group is critical for this aqueous aerobic chemistry. In turn, the oxidation products (dpms)PtIVR(OH)2 eliminate cleanly methanol (R=Me) or epoxide (R=olefin-derived) in water. Migration of hydrocarbyl in (dpms)PtIVR(X)OH from an equatorial to the axial position is an important step of these C-O elimination reactions. The isomers having the sulfonate trans- to the alkyl react dramatically faster.
1. Unlike previously known monohydrocarbyl PtII complexes, (dpms)PtIIR(HX) (dpms = di-2-pyridylmethanesulfonate; HX=H2O, MeOH, EtOH; R=Me, HOC2H4 etc) can be readily oxidized by O2 at room temperature to produce (dpms)PtIVR(X)OH in quantitative yield (Scheme 1). A number of olefin complexes (dpms)PtII(olefin)OH undergo facile aerobic oxidation in water to produce PtIV alkyls (olefin=C2H4, cyclooctene, norbornene). We found that anionic intermediates (dpms)PtIIR(X)- are the key species responsible for the reaction with O2. The presence of the sulfonate group is critical for this aqueous aerobic chemistry. In turn, the oxidation products (dpms)PtIVR(OH)2 eliminate cleanly methanol (R=Me) or epoxide (R=olefin-derived) in water. Migration of hydrocarbyl in (dpms)PtIVR(X)OH from an equatorial to the axial position is an important step of these C-O elimination reactions. The isomers having the sulfonate trans- to the alkyl react dramatically faster.
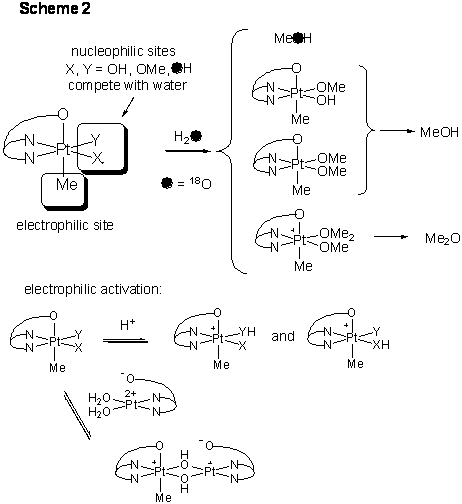 Using kinetic and isotopic labeling experiments we discovered a novel mechanism of C-O elimination from PtIV center. It involves nucleophilic attack of a hydroxo ligand of LPtIVMe(OH)2 at the methyl carbon of electrophilic LPtIVMe(OH)2·H+ cation. The reaction order in Pt is two; PtIV methoxo intermediates and dimethyl ether are observed. This process competes with the reaction involving water as a nucleophile (Scheme 2).
Using kinetic and isotopic labeling experiments we discovered a novel mechanism of C-O elimination from PtIV center. It involves nucleophilic attack of a hydroxo ligand of LPtIVMe(OH)2 at the methyl carbon of electrophilic LPtIVMe(OH)2·H+ cation. The reaction order in Pt is two; PtIV methoxo intermediates and dimethyl ether are observed. This process competes with the reaction involving water as a nucleophile (Scheme 2). 
Similar to dpms complexes, aerobic oxidation of (Me2Bpy2)PtIIR(X)- (R=Me, Ph; X=R, OMe) is also possible in water or alcohols. Not only oxidation of PtII to PtIV but also unprecedented clean B-to-PtIV hydrocarbyl transfer are observed (Scheme 4). We suggest that CH agostic PtIV complexes might be the reaction intermediates. A model CH agostic complex (Me2Bpy2)PtIVMe3 was isolated and characterized in detail including X-ray crystallography. 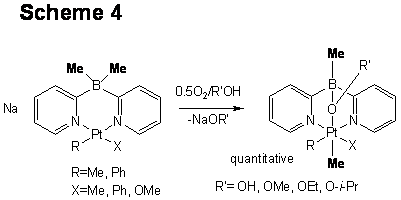
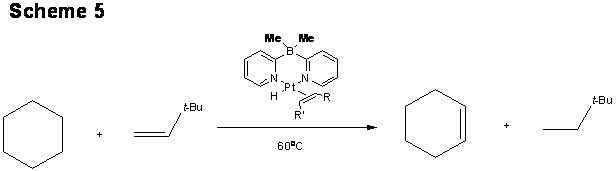
Using (Me2Bpy2)PtH(olefin) as a catalyst, we developed first Pt-based mild catalytic alkane transfer dehydrogenation utilizing tert-butyl ethylene as a sacrificial olefin (Scheme 5).