
ACS PRF | ACS
All e-Annual Reports

42806-B3
Compounds of Bidentate Phosphines with Metallocene Backbones: Electrochemistry and Catalysis
The undergraduates in my lab have continued to investigate the properties of bidentate phosphines that have metallocene backbones. To date our work has focused on the synthesis, electrochemistry and structural analysis of compounds containing either
1,1'-bis(diphenylphosphino)metallocene (metallocene = ferrocene (dppf), ruthenocene (dppr) or osmocene (dppo)) of 1,1'-bis(dialkylphosphino)ferrocene (alkyl = i-propyl (dippf) or t-butyl (dtbpf)). With the exception of dtbpf, the oxidative electrochemistry of the free ligands is complicated by a chemical reaction.
We recently investigated the oxidative electrochemistry of the free ligand 1,1'-bis(dicyclohexylphosphino)ferrocene (dcpf) and complexes containing this ligand.1 As with the similar ligands, the oxidative electrochemistry of the free ligand is followed by a chemical reaction while oxidation of compounds containing dcpf is typically reversible. In addition, the structure of [(dcpf)PdCl2] was determined (Figure 1). The P-Pd-P angle in [(dcpf)PdCl2] was determined to be 102.45o which is larger than the dppf analogue and smaller than the dippf analogue.
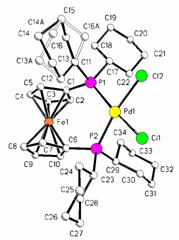
Figure 1. X-ray structure of [(dcpf)PdCl2].
The use of the [(P-P)PdCl2] (P-P = dppf, dppr, dippf, dcpf or dtbpf) compounds as catalyst precursors in Buchwald-Hartwig catalysis was also investigated.1 Previous studies had employed several of the compounds, but there have been no reports comparing all of these ligands for the same catalytic reactions. We investigated the coupling of a primary amine with an aryl bromide (Scheme 1). In general, we found the dppf compound to be the most effective catalyst, although with the electron withdrawing

Scheme 1. Buchwald-Hartwig reaction.
-CF3 group, the dtbpf gave the most efficient catalyst. Additional catalytic studies are currently underway.
We have also developed an interest in the closely related chiral bidentate phosphines with metallocene backbones. Our initial work was with the BoPhoz ligands.2 More recently, we investigated the oxidative electrochemistry of a series of Josiphos ligands (Figure 3).3 The oxidative electrochemistry typically displays multiple waves with varying amounts of reversibility. The initial oxidation seems to occur at the phosphorus atoms bound directly to the C5 ring. Subsequent oxidations occur likely occur in the π-system and at the iron center. In addition to the free ligands, we investigated the oxidative electrochemistry of the [(Josiphos)PdCl2] and [(Josiphos)PtCl2]. Upon coordination the electrochemistry simplifies to one reversible peak.
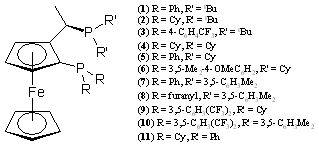
Figure 3. Josiphos ligands.
1) Hagopian, L. E.; Campbell, A. N.; Golen, J. A.; Rheingold, A. L.; Nataro, C. J. Organomet. Chem. 2006, 691, 4890.
2) Ghent, B. L.; Sites, L. A.; Rheingold, A. L.; Nataro, C. Organometallics 2005, 24, 4788.
3) Ghent, B. L.; Martinak, S. L.; Sites, L. A.; Golen, J. A.; Rheingold, A. L.; Nataro, C. J. Organomet. Chem. 2007, 692, 2365.