
ACS PRF | ACS
All e-Annual Reports

41360-B4
Ring Strain and Antiaromaticity: Their Effect on the Generation and Reactivity of Enolates in Small-Ringed Ketones
The fundamental importance of enolates as intermediates in the formation of carbon-carbon bonds is obvious when one considers the amount of space devoted to this topic in Organic Chemistry textbooks. An area of enolate chemistry that has not received as much attention due to the challenges associated with their study is the effect of ring strain on the generation and reactivity of enolates in cyclic ketones. The strained ring ketones are fundamentally interesting as the induction of strain will result in increased s-character in the C-H bonds thus increasing their acidity, however the ring strain should disfavor the generation of the enolate which would introduce another sp2-hybridized carbon into the ring. In addition, the study of the reactivity of these strained ring ketones, with respect to enolate chemistry, could have implications in our understanding of many other reactions where strain has been proposed to play a role in the reactivity of the system. An example, would be the induction of strain in substrate molecules and the reduction of degrees of rotational freedom that are often proposed as important modes of lowering the activation energy of enzymatically catalyzed reactions. The ketones in which we are interested have varying degrees of strain and the a-protons have reduced mobility due to the ring.
The proposed studies had four fundamental parts and each of these sections will be addressed independently.
i) Determining the pKa of Benzocyclobutenone (1): Previously, we had determined the rate of the quinuclidine-catalyzed (pKBH = 11.5) deprotonation of 1 to be kB = 7.2 x 10-6 M-1 s-1in D2O, at 25oC and I = 1.0 (KCl). In addition the rate of the 3-quinuclidinol (pKBH = 10.0) catalyzed reaction was found to be kB = 4.8 x 10-7 M-1s-1 in D2O, at 25oC and I = 1.0 (KCl).
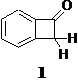

ii) Reactivity Studies of Substituted Benzocyclobutenone Derivatives: During the Summer of 2007, Rick Yarbrough (Undergraduate, Iraqi war veteran) continued our work in this area. The goal was to build a small library of benzocyclobutenone derivatives so that the reactivity of the a-protons could be investigated. The synthetic scheme outlined above has produced a number of derivatives that have been isolated and purified. However, in some instances mixtures of regioisomers are produced that lead to inseparable mixtures of the desired compounds. As a result, significant time was invested in probing others synthetic methods of producing these compounds in an effort to minimize or eliminate the production of multiple regioisomers.
iii) Determination of the pKa of Cyclobutanone: We have performed deuterium incorporation studies on cyclobutanone and based upon the second-order rate constants for general-base catalyzed determined the pKa of cyclobutanone to be 20.0. This aspect of the project then began to develop as continued our deuterium incorporation studies with the lesser strained cyclopentanone system. Previous to our investigations of cyclopentanone, all deuterium incorporation studies involving enolate intermediates relied heavily on results from studies involving acetone. However, the rate constants for enolate formation in cyclobutanone were not significantly different than those obtained for acetone. This led to questions concerning the effect of the carbocycle restricting the orientation of the a-protons with regard to the carbonyl group. The pKa of cyclopentanone was estimated to be 18.5, which is considerably more acidic than both acetone and cyclobutanone. These results led to further difficulties because in all cases cyclopentanone reacted more quickly than cyclobutanone. Previous studies found that the relative rate of enolization were C4 > C5 > C6 > C7 ~ 4-heptanone or C4 > C5 > C8 > C7 > C6.
We were beginning to see trends in the reactivity of these cyclic compounds that were beginning to shed light on the role of both ring-strain and the effects of the carbocycle restricting C-C rotational freedom. We have expanded this project to include cyclohexanone and 3-pentanone. Early results indicate that cyclohexanone is not as reactive as cyclopentanone but is more reactive than cyclobutanone.
iv) Effect of Increasing Ring Strain on the Reactivity of ?-Protons: The synthesis of bicyclo[4.2.0]octan-7-one and bicyclo[3.2.0]heptan-6-one was accomplished however the bridgehead protons complicated the 1H-NMR spectra. Two methods were developed to overcome this problem: a) Synthesize cyclohexene where the alkene carbons were deuterated. b) Synthesize 1,2-dimethylcyclohexene. Both of these compounds can be used for the 2 + 2 cycloaddition product. The deuterated cyclohexene was synthesized directly before Mr. Cope's graduation and has not proceeded any further. The synthesis of 1,2-dimethylcyclohexene has currently proceeded to 2,7-octadione which now needs to be undergo McMurry coupling to form the alkene to be used in the cycloaddition reaction.