
ACS PRF | ACS
All e-Annual Reports

42701-G5
Gas-Phase Heterogeneous Catalysis on Metal Nanoparticles: Size-Dependence
During the second year of ACS-PRF funding, we have investigated the effect of the nanoparticle support and the oxidation state of the Pt nanocatalysts on their activity and selectivity for the decomposition of methanol (MeOH). This process has increased in significance due to its applicability as a storage fuel for hydrogen and subsequent use in fuel cells. In addition, methanol's adaptability to the existing infrastructure (as a liquid fuel) makes it attractive for transportation applications.
There is a current debate in the literature on whether metallic Pt or Pt oxides are the most catalytically active species. In the first year of this grant, we have shown that Pt nanoparticles (NPs) supported on TiO2 have a size-dependent activity in the decomposition of MeOH, where an increase in activity was associated with a decrease in particle size. Furthermore, the oxidation state of the nanoparticles was found to depend on the initial size of the particles. Although support related effects were not considered owing to the use of a common support, they were assumed to be present and are in need of consideration.
For this study, size-selected Pt nanoparticles were prepared by encapsulation in diblock-copolymer micelles (PS-P2VP) and were deposited on a series of reducible (TiO2, CeO2) and non-reducible (SiO2, Al2O3 and ZrO2) nanocrystalline supports. Figure 1 displays AFM (a) and TEM (b),(c) images of our nanocatalysts.
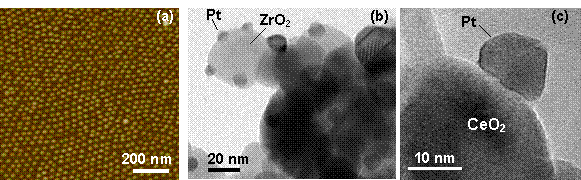
Fig. 1 AFM (a) and (b), (c) TEM images of Pt nanoparticles supported on SiO2/Si(001), ZrO2, and CeO2, respectively.
Although Pt NPs with identical average sizes were deposited on all supports, the choice of support was found to strongly influence their stability against coarsening during polymer removal by annealing in air at 500°C. Two different Pt size distributions were obtained: ~ 15-18 nm for Pt/CeO2 and Pt/SiO2, and ~ 8-9 nm for Pt/TiO2, Pt/ZrO2, and Pt/Al2O3.
The oxidation state of Pt was found to depend on the NP support used. Fig. 2 shows X-ray photoelectron spectroscopy (XPS) data from Pt NPs deposited on different powder oxide supports. The solid lines indicate the positions of the main core-level peaks of metallic Pt, the dashed lines Pt2+ in PtO, and the dotted lines Pt4+ in PtO2. The Pt/TiO2 sample was found to be predominantly metallic. However, for Pt/ZrO2 and Pt/SiO2, a convolution of Pt2+ and Pt4+ was observed, with the former being mainly Pt2+ and the latter slightly favoring contributions from Pt4+. The Pt/CeO2 sample appears highly oxidized (mainly Pt4+) and the higher binding energies indicate a strong interaction between the CeO2 support and the Pt particles. The possible formation of Pt-Ce alloys might explain the anomalously large binding energies observed.
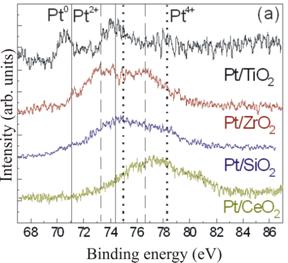 Fig. 2 Pt-4f core level XPS spectra of Pt nanoparticles supported on TiO2, ZrO2, SiO2, and CeO2. All spectra were measured after removal of the encapsulating polymer by annealing in air at 500°C.
Fig. 2 Pt-4f core level XPS spectra of Pt nanoparticles supported on TiO2, ZrO2, SiO2, and CeO2. All spectra were measured after removal of the encapsulating polymer by annealing in air at 500°C.
Reactivity studies at atmospheric pressure were conducted in a packed-bed mass flow reactor interfaced to a quadrupole mass spectrometer, Fig. 3. The Pt/ZrO2 system was found to be the most active, with nearly 100 % conversion at 250°C. Interestingly, the Pt NPs in the Pt/ZrO2 sample were found to be in a cationic state. Further, the selectivity towards H2 was found to increase significantly with respect to the Pt-free supports, since the main products detected were H2 and CO. The poor performance of the Pt/CeO2 system (Fig. 3) is explained in terms of an enhanced NP sintering upon thermal pre-treatment. Our data suggest that the most important parameters to achieve an effective and selective MeOH decomposition are the size and the oxidation state of the Pt nanocatalysts. The role of the support is that of a stabilizer, a provider of preferential/additional sites of interaction, and a mediator among the different oxides of Pt. The above work has stimulated the recruitment of talented undergraduate and graduate students and has served as a training platform for K12 interns.

Fig. 3 MeOH decomposition over Pt nanoparticles supported on ZrO2, Al2O3, TiO2, CeO2, and SiO2.