ACS PRF | ACS
All e-Annual Reports

42158-G4
Serially Addressable Fusion Protein-Tag System
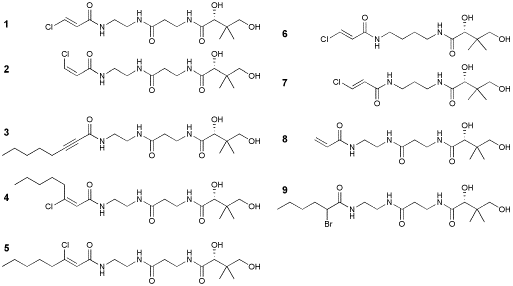
In both fatty acid and polyketide synthases, tight, but transient, interaction between the ketosynthase domain and the acyl carrier protein is essential for the efficient productivity. The carrier protein with bound substrate must first recognize the binding domain of its partner ketosynthase domains; the complementary structures and surface charges on these proteins drive this process. While the architecture of the carrier protein does change slightly upon modification of its conserved serine residue, the overall fold and positions of surface residues of the protein do not change significantly. Therefore, if the two proteins can recognize one another, they will only interact when the carrier protein is carrying an acceptable substrate. Here we report the further results of our crosslinking studies with our 3 KS-ACP systems: E. coli KASI-ACP and KASII-ACP and S. maritimus EncAB-EncC. We verify the active site specificity of our crosslinking experiments by performing MALDI and LC-MS-MS on in-gel-digested crosslinked proteins. We explore through SDS-Page experiments the interactions between both natural and unnatural carrier protein and ketosynthase partners using our panel of reactive pantetheine analogs. We show our results reflect the specificity of a ketosynthase for its natural ACP substrates. We also show that our system accurately mirrors the differences in both binding affinities and substrate specificity between the ketosynthases. In addition, we draw new insight into the interaction between ACP's and ketosynthases in these biosynthetic systems. The nine pantetheine analogs in our panel contain reactive moeities that act as substrate mimics for ketosynthase domains. These pantetheine-like compounds bound to ACP act as either Michael acceptors (compounds 1-8) or alpha-halo alkylating agents (compound 9). Post-translational modification of the apo-form of the carrier proteins E. coli AcpP and S. maritimus EncC by our panel of pantetheine analogs yielded reactive crypto-ACP's. Each crypto-ACP then was reacted with its natural partner ketosynthase. Two of the more potent crypto-ACP forms were also incubated with a ketosynthase both from a different organism and biosynthetic system. In almost all cases tested there was some interaction between crypto-ACP and ketosynthase. This interaction was detected as a gel shift on SDS-Page; the degree of crosslinking was quantified using the gel analyzing function of the program ImageJ. For all natural systems, crosslinking was confirmed by MADLI and LC-MS-MS analyses. The site-specific nature of our crosslinking system between E. coli ACP and both KASI and KASII was previously demonstrated using data from MALDI MS/MS analyses of in gel digested complexes. We extended this approach to the S. maritimus carrier protein EncC and ketosynthase EncAB, which are involved in the biosynthesis of the polyketide enterocin. EncC was post-translationally modified by a CoA entity derived from compound 3. Crypto-EncC was allowed to react with EncAB. The one-pot reaction was then run on multiple lanes of SDS-Page; the subunits EncA (KS) and EncB (chain length factor) of the ketosynthase were dissociated under these denaturing conditions. Bands corresponding to crosslinked EncA-EncC were then excised and digested with either trypsin, chymotrypsin, or pepsin. MALDI MS/MS analyses resulted in the identification of peptides corresponding to 71% sequence coverage of EncC and 96% of EncA. Importantly, the active site residues, Ser72 of our EncC construct and Cys191 of our EncA construct, were not identified in the analysis. This suggests post-translational modification of Ser72 and subsequent reaction of crypto-EncC with Cys191, resulting in the site-directed crosslink between EncAB and EncC. To assess the functional interaction of ketosynthases with substrate-bound carrier proteins, we performed 10 one-pot crosslinking reactions for each system tested. In each case, the carrier protein was first allowed to react with the pantetheine analog in the presence of CoA biosynthetic enzymes and the phosphopantetheinyl-transferase Sfp. After an initial incubation, the modified CP was reacted with the appropriate ketosynthase. The reaction proceeded and the entire one-pot reaction was loaded onto SDS-Page. All reactions were performed in parallel at the same time to ensure that all variables were controlled for. Following SDS-Page, the gels were stained and photographed. The gel images were then analyzed by the ImageJ program provided by NIH. Our results agree with a variety of studies that investigated substrate specificity and protein interactions of ketosynthases and carrier proteins. We established the site-specificity of our crosslinking agents in the natural KASI-ACP, KASII-ACP, and EncAB-EncC systems. The site-specificity of the crosslinks between unnatural partner proteins was not evaluated in this study. Future studies exploring the functional aspects of these unnatural interactions will address this issue. Our tools thus established can aid the development of unnatural biosynthetic systems. With mutagenesis, we could use our system to determine the amino acid residues important for protein-protein and enzyme-substrate interactions in these systems. In addition, a larger and more diverse panel of analogs would give us further incite into substrate recognition and the potential activity of unnatural biosynthetic systems.