ACS PRF | ACS
All e-Annual Reports

42056-AC2
Ion Mobility on Calcite Surfaces
Our goal is to understand the relationship between mobile ions and calcite (CaCO3) surface reconstruction. With recent results, we established that there is a tight coupling between mobile-ion density and the progress of surface reconstruction. At relative humidity around 80%, films of 1to 2 nm high form on the (104) surface as a result of surface reconstruction. We characterized the impact of the formation of these nano-films on calcite surface electrostatics, in terms of surface potential and its relationship with ion mobility. Surface potential is a composite quantity linked to the local lattice structure of the reconstructed surface, the adsorption of water layers, and the mobile surface ions formed by dissolution.
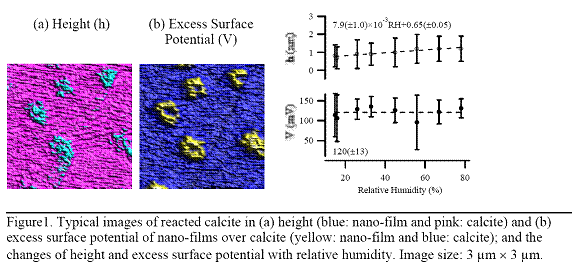
We grew the nano-films on freshly cleaved calcite surface at 80% RH in nitrogen. After 3 hr of reaction, the nano-films were identified by their morphology and height using atomic force microscopy (AFM). Surface potential of the reacted surface was then characterized using Kelvin probe force microscopy (KPFM). Results are highlighted in Figure 1.
The observations measured using KPFM, a technique sensitive to differences in surface potential but filters out differences in height, show that the nano-films of only 1 nm high have a surface potential that is 126(±31) mV higher than that of calcite. This difference, referred to as excess surface potential, remains virtually constant with increasing relative humidity while the height of the nano-films increase from 0.8(±0.4) nm at 15% RH to 1.3(±0.5) at 78% RH (67% increase).
Both the increasing height and the constant excess surface potential can be related to the chemical composition of the nano-films. The nano-films are believed to be calcium carbonate with water loosely bonded to its lattice (CaCO3·xH2O). With increasing humidity, the adsorption of water molecules increases on the reacted surface. Because water is part of nano-film structure, their height increases with the increase of the amount of adsorbed water. Calcite and CaCO3·xH2O dissolve into the adsorbed water layers and form surface mobile ions. We demonstrated that the density of mobile ions goes up with increasing RH and that the mobile ions in turn increase the absolute value of calcite surface potential. The solubility of CaCO3·xH2O is expected to be similar to that of calcite. Hence, the effect of RH on water adsorption, ions release, and the resulting change of surface potential should be similar for both the nano-films and the calcite substrate. Consequently, the excess surface potential, which is the surface-potential difference between the nano-films and calcite, remains constant with increasing RH.
The results of this study for humid air provide insights into aqueous-phase properties because the water structures near calcite surface are similar in both environments. The excess surface potential measured with the first layer of adsorbed water at ca. 50% RH in air may therefore also represent the zeta-potential difference between the nano-films and calcite at the stern layer in aqueous solution. Along these lines, the measured results at 80% RH may represent conditions further away in the diffuse layer. Increased surface potential in the aqueous phase can increase the adsorption of negatively charged contaminants and microorganisms and reduce the adsorption of their positively charged counterparts.
Impact on the Students who Participated in the Project The student co-authors on this project have go on to faculty positions: Treavor Kendall (Clemson University) and Young-Shin Jun (Washington University).