
ACS PRF | ACS
All e-Annual Reports

45128-G5
Investigation of the Fundamental Properties of Partition Layers in Surface-Enhanced Raman Scattering
The goal of this PRF is to generalize the SERS technique by exploring the use of Ag substrate-bound partition layers to preconcentrate low affinity analytes within the zone of electromagnetic enhancement. During year 1, progress toward this goal has been made on two fronts: the implementation of a novel SERS substrate and studies of how partition layer hydrophobicity influences analyte detection.
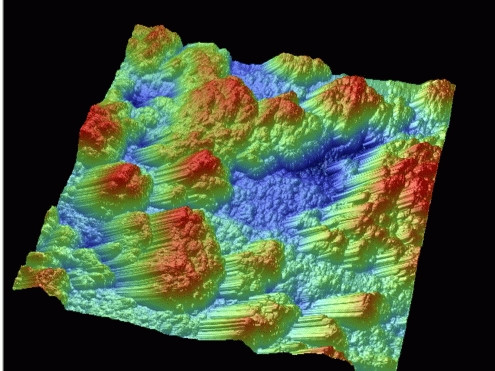 When designing a SERS substrate, it is advantageous to maximize surface area, achieve good signal stability in a myriad of solvents, and attain low background fluorescence. Toward this end, the Haynes group has recently developed polymer-templated electroless deposition SERS substrates using poly(diallyldimethylammonium chloride). Fabrication begins by making a polymer/nanoparticle solution which is deposited onto a glass coverslip. After drying, the film is placed into a Ag electroless plating bath where island growth initiates at the embedded nanoparticles and progresses through the polymer film. The final substrate feature size is determined by the nanoparticle loading density, the ionic strength of polymer solution (because this determines the globular or extended nature of the polymer chains), and the electroless deposition time. To date, variations have been made independently in all of these parameters, yielding a wide variety of nanostructured substrates, and accordingly, substrate optical properties. For example, upon varying the [NaCl] in the polymer deposition solution from 0.001 to 1 M in 10-fold increments, the AFM-measured Ag island area increases 10% for each 100 mM increase in [NaCl] (p<0.0005). This indicates that higher ionic strength promotes extension of the polymer chain. An AFM image of a substrate is shown in Figure 1. Each of the substrate's SERS potential was assessed by adsorbing a monolayer of benzenethiol and measuring a SERS spectrum. Enhancement factors were calculated for each substrate by comparing the signal intensity achieved to that measured as normal Raman scattering from liquid benzenethiol (while accounting for the differing number of scatterers present in each measurement). In all cases, enhancement factors of greater than 106 were achieved on the polymer-templated substrates; this compares favorably to the enhancement factor of 6 x 105 measured from a traditional SERS substrate, the Ag film over nanospheres. The new substrate has further advantages – specifically, it can be generated without expensive and equipment-intensive vapor, electron-beam, or electrochemical deposition and these substrates are stable in aqueous environments.
When designing a SERS substrate, it is advantageous to maximize surface area, achieve good signal stability in a myriad of solvents, and attain low background fluorescence. Toward this end, the Haynes group has recently developed polymer-templated electroless deposition SERS substrates using poly(diallyldimethylammonium chloride). Fabrication begins by making a polymer/nanoparticle solution which is deposited onto a glass coverslip. After drying, the film is placed into a Ag electroless plating bath where island growth initiates at the embedded nanoparticles and progresses through the polymer film. The final substrate feature size is determined by the nanoparticle loading density, the ionic strength of polymer solution (because this determines the globular or extended nature of the polymer chains), and the electroless deposition time. To date, variations have been made independently in all of these parameters, yielding a wide variety of nanostructured substrates, and accordingly, substrate optical properties. For example, upon varying the [NaCl] in the polymer deposition solution from 0.001 to 1 M in 10-fold increments, the AFM-measured Ag island area increases 10% for each 100 mM increase in [NaCl] (p<0.0005). This indicates that higher ionic strength promotes extension of the polymer chain. An AFM image of a substrate is shown in Figure 1. Each of the substrate's SERS potential was assessed by adsorbing a monolayer of benzenethiol and measuring a SERS spectrum. Enhancement factors were calculated for each substrate by comparing the signal intensity achieved to that measured as normal Raman scattering from liquid benzenethiol (while accounting for the differing number of scatterers present in each measurement). In all cases, enhancement factors of greater than 106 were achieved on the polymer-templated substrates; this compares favorably to the enhancement factor of 6 x 105 measured from a traditional SERS substrate, the Ag film over nanospheres. The new substrate has further advantages – specifically, it can be generated without expensive and equipment-intensive vapor, electron-beam, or electrochemical deposition and these substrates are stable in aqueous environments.
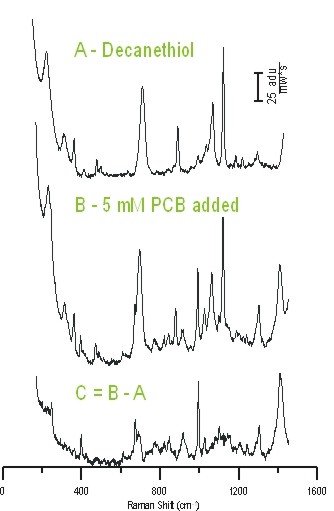 Following development of a cheap, stable SERS substrate with high enhancement factors, these substrates were implemented for partition layer studies. Initial work has focused on how partition layer hydrophobicity influences detection of 2,2',4,4'-tetrachlorobiphenyl (TCB), a molecular species with no natural affinity for Ag or Au substrates, and thus, a traditionally inaccessible detection target for SERS. In this work, partition layers were assembled from decanethiol and perfluorodecanethiol solutions. To date, it is possible to detect 1 mM TCB that has partitioned into both the decanethiol and perfluorodecanethiol layers (see Figure 2). In general, the SERS signal intensity is larger when decanethiol is used though the source of this improved signal may be either more efficient TCB partitioning or a better match between the excitation laser and the substrate optical properties. Currently, work is underway to use a reflectivity probe to monitor the optical properties of these new non-transmissive SERS substrates. Based on the extremely hydrophobic nature of the fluorinated partition layer, the solvent refractive index effects may shift the substrate's optical properties significantly. Further work is focused on determining the TCB limit of detection with both partition layers. SERS spectra are information rich, and spectra collected to date demonstrate a significant shift in the Ag-S stretch when using both partition layer molecules (an 18 cm-1 shift with decanethiol and a 13 cm-1 shift with perfluorodecanethiol). This shift indicates that the TCB is partitioning into the monolayer, disturbing the original packing density, and not merely collecting at the monolayer/solution interface. The larger shift in the case of decanethiol may indicate a higher loading or deeper penetration of TCB into the layer; further analysis of the C-C band shifts, as well as limit of detection calculations, will facilitate distinction between these two possibilities. In addition, the presence of the low frequency C-Cl stretch bands indicates that the TCB orientation in the partition layer is not completely parallel with the substrate.
Following development of a cheap, stable SERS substrate with high enhancement factors, these substrates were implemented for partition layer studies. Initial work has focused on how partition layer hydrophobicity influences detection of 2,2',4,4'-tetrachlorobiphenyl (TCB), a molecular species with no natural affinity for Ag or Au substrates, and thus, a traditionally inaccessible detection target for SERS. In this work, partition layers were assembled from decanethiol and perfluorodecanethiol solutions. To date, it is possible to detect 1 mM TCB that has partitioned into both the decanethiol and perfluorodecanethiol layers (see Figure 2). In general, the SERS signal intensity is larger when decanethiol is used though the source of this improved signal may be either more efficient TCB partitioning or a better match between the excitation laser and the substrate optical properties. Currently, work is underway to use a reflectivity probe to monitor the optical properties of these new non-transmissive SERS substrates. Based on the extremely hydrophobic nature of the fluorinated partition layer, the solvent refractive index effects may shift the substrate's optical properties significantly. Further work is focused on determining the TCB limit of detection with both partition layers. SERS spectra are information rich, and spectra collected to date demonstrate a significant shift in the Ag-S stretch when using both partition layer molecules (an 18 cm-1 shift with decanethiol and a 13 cm-1 shift with perfluorodecanethiol). This shift indicates that the TCB is partitioning into the monolayer, disturbing the original packing density, and not merely collecting at the monolayer/solution interface. The larger shift in the case of decanethiol may indicate a higher loading or deeper penetration of TCB into the layer; further analysis of the C-C band shifts, as well as limit of detection calculations, will facilitate distinction between these two possibilities. In addition, the presence of the low frequency C-Cl stretch bands indicates that the TCB orientation in the partition layer is not completely parallel with the substrate.
In conclusion, significant progress has been achieved in year 1 of this PRF-sponsored research. In the coming year, effort will be focused on characterizing the optical properties of the polymer-templated SERS substrates before and after partition layer adsorption, implementing both alkanethiol and perfluoroalkanethiol partition layers of varying length to optimize TCB loading and the achieved SERS signal, examining hydrophilic partition layers such as thiolated poly(ethylene glycol), implementing a flow cell to watch TCB partitioning kinetics, assessing departitioning from the substrates (i.e. reusability), and using optimized substrate/partition layers to detect a target analyte from a complex mixture of similar chemical species.