ACS PRF | ACS
All e-Annual Reports

42561-AC10
Metals in Negative Oxidation States
This project continues our endeavour to discover new polar intermetallic compounds containing anionic networks of the heavier p-block and late d-block metals or metalloids (X), such as In, Ge, Sn, Bi, Au, and Hg, in combination with electropositive components, viz. alkali (A), alkaline-earth (AE), and rare-earth metals (R).
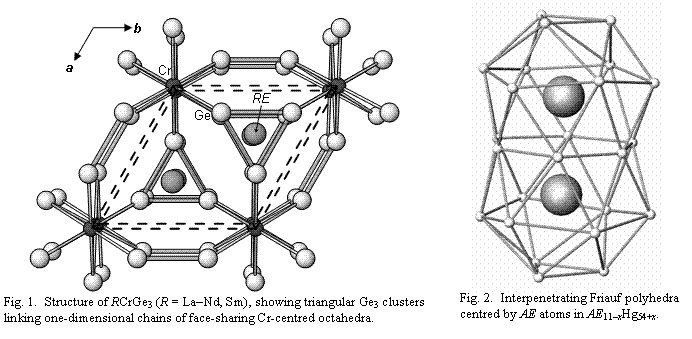
Anionic networks of the heavier p-block elements. Expanding on our long-established expertise on ternary rare-earth transition-metal antimonides R-M-Sb, we have been interested in new systems involving other heavier p-block elements. Because Ge and Sb have similar electronegativities and atomic radii, exemplifying what might be regarded as a diagonal relationship in the periodic table, the bonding character in ternary germanides will resemble that of the antimonides, suggesting that anionic Ge-Ge networks might also be formed. We have systematically investigated the phase diagrams of several R-M-Ge systems, where M is an early transition metal such as Ti, V, or Cr. A new family of ternary germanides with the composition RCrGe3 has been identified, adopting a hexagonal perovskite-type structure, which is normally found for halides or chalcogenides and is unusual for an intermetallic compound. Whereas isolated X1- or Q2- species can be regarded to be present in the more ionic representatives, the same cannot be said for RCrGe3, which must necessarily have a polyanionic structure. Consideration of simple electron-counting rules suggests the formation of two Ge-Ge bonds per atom, which is realized in the structure in the form of triangular Ge3 clusters (Fig. 1). These compounds are ferromagnetic metals with relatively high ordering temperatures (Tc ranging from 60 K to 155 K). Solid solutions in which Cr is disordered with other first-row transition metals are being prepared to study the effect on the electrical and magnetic properties. Other new phases discovered include the isostructural vanadium series, RVGe3, and another Ge-rich series, RCrGe2, containing zigzag Ge chains. A mixed Ge-Sb series has also been identified, R12Ge7Sb21, showing a variety of Ge-Ge, Ge-Sb, and Sb-Sb bonding interactions.
Aurides and mercurides. Binary phases of Au or Hg in combination with A or AE metals are quite challenging to prepare, given the complexity of their phase diagrams and the air-sensitivity of the crystals. Even when we have successfully identified a new compound, the difficulties are magnified by the extreme absorption problems in the course of analyzing single-crystal X-ray diffraction data. Overcoming these challenges, we have re-examined the mercury-rich regions of the Ca-Hg and Sr-Hg phase diagrams and shown that the phases previously identified as "AEHg3.6" should be reformulated as AE11-xHg54+x (AE = Ca, Sr). The homogeneity range is small, with theoretical limits of 0 < x < 2 for AE11-xHg54+x, corresponding to "AE0.14-0.17Hg0.86-0.83". The structure is extremely complex, consisting of a dense packing of atoms in diverse coordination polyhedra (CN11-16). For example, a pair of Friauf polyhedra with AE atoms at the centres and Hg atoms at the vertices forms a key cluster motif (Fig. 2). The structure can also be regarded as being built up from a stacking of triangular nets with hexagonal voids filled by single atoms or various clusters, such as a triangular Hg3 unit. Band structure calculations confirm that a small degree of electron transfer takes place to the Hg atoms, supporting the presence of a partially anionic mercuride substructure. Having mastered the techniques necessary to handle these highly air-sensitive phases and applying rigorous absorption corrections to refine their crystal structures accurately, we have now been blessed with an embarrassment of riches. New binary and ternary phases that we have prepared and whose structures we are currently refining include: Li6Ca17Hg9, Li6Sr17Hg9, Li6Yb17Hg9, LiCa3Hg, KIn8Hg2, Na29Ag24Sn38, LiHgSn2, CaHgSb2, Li2Hg2Sb, LiHg3Sn, Na2Hg6In5, NaHgSn, and SrHg13.