
Back to Table of Contents
44644-AC3
A New Twist on Pincer Complexes for Catalysis
John Protasiewicz, Case Western Reserve University
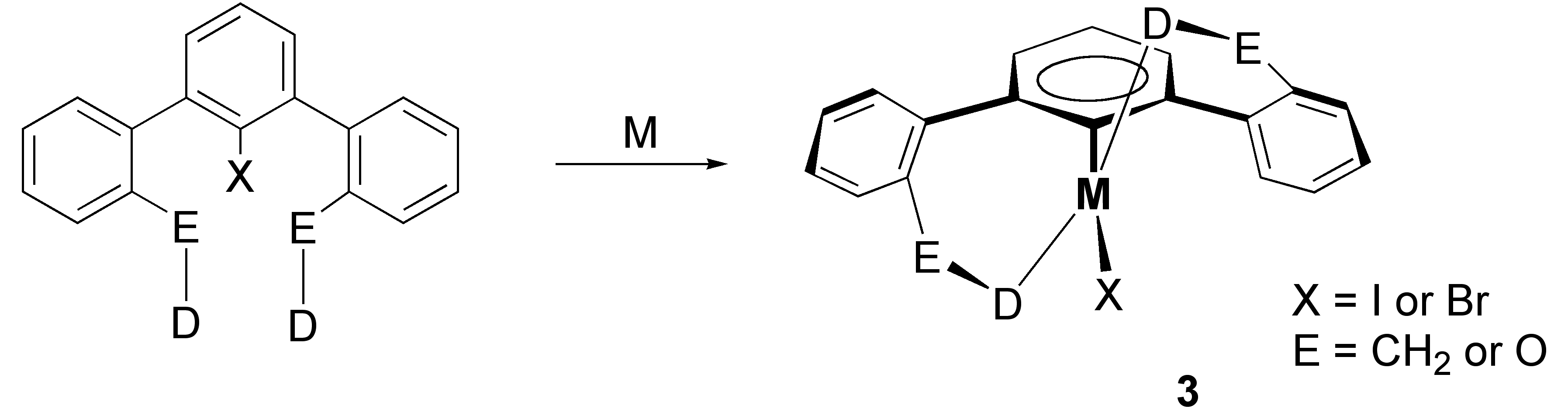
During the first funding period of this award, we have greatly expanded the types of metal-ligand complexes that bear the new pincer ligands (Chart 1). Originally, we found ligand precursors 2a-2d and 2m could be synthesized. We can now report on the synthesis of 2e, 2j-2l, and 2n. Efforts to prepared the remaining ligand precursors are underway.
Chart 1.

The new ligand precursors were found to react with low valent palladium and nickel reagents to give the desired pincer type complexes (Scheme 1).
Scheme 1.
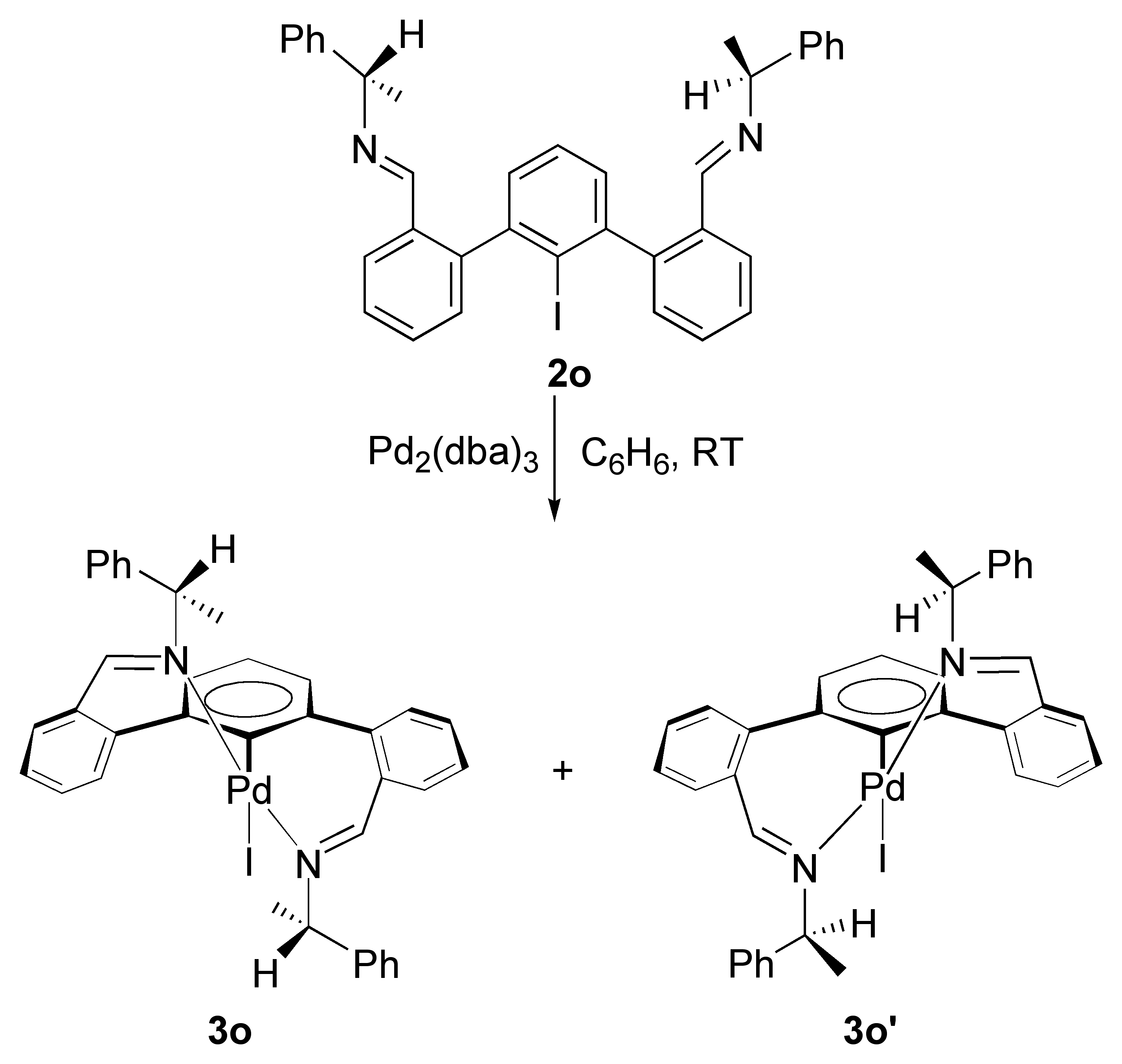
Two papers have been published having different metal complexes. The first that has appeared in Inorganic Chemistry as a full paper describes new palladium diimine complexes 3j-3l. A large number of these complexes have been structurally characterized and all have a similar structure as portrayed above. One very exciting finding of this work was that we were successful in isolating individual diastereomers of such complexes (3o and 3o‰Ûª) by using an enantiomerically pure amine for forming the diimine ligand precursor (Scheme 2).
Scheme 2.
We are also exploring the catalytic potential of our new complexes. Ligand 2n and its palladium complex 3n have proved to be singularly competent catalyst precursors for the Suzuki-Miyaura coupling of aryl halides with aryl boronic acids (Scheme 3).
Scheme 3.

For example, complex 3n can promote the catalytic coupling 2-chloroanisole with p-tolyl boronic acid in 82% yield (isolated) at 75°C. Such results are impressive, as many previous studies have required higher temperatures (100°C or greater) and often do not work for aryl chlorides (but can convert more reactive aryl bromides and aryl iodides).
Back to top





