ACS PRF | ACS
All e-Annual Reports

38948-AC7
Modified Fullerene Vesicles and Membranes: Supramolecular Structures, Dynamics and Interactions
Surfactants including their biological variation known as lipids are amphiphilic molecules having both hydrophilic and hydrophobic molecule parts covalently bonded in the same molecule. One way to form a hydrophilic head group is to make it electrostatically charged (for a non-charged alternative see non-ionic surfactants). The sign of the electrostatic charge determines with which types of polyelectrolytes, i.e., polymers with oppositely charged ionic groups, these surfactants can form complexes. Anionic surfactants, e.g., those having carboxylic, sulfonic or phosphoric acid groups, can form complexes with cationic polyelectrolytes, and cationic surfactants (essentially all of them carrying organically substituted ammonium groups) can form complexes with anionic polyelectrolytes. Since the polyelectrolyte we are ultimately interested in, DNA, is anionic the surfactants investigated in this study are cationic surfactants.
In traditional surfactants and also in lipids, the hydrophobic part of the molecule is formed by one or more aliphatic chains of typically 10 to 20 carbon atoms length. Cationic chain-based surfactants are known to be quite toxic which limits their use as therapeutic agents. A considerably less toxic alternative has been found in fullerene-based cationic surfactants where hydrophobic part of the molecule is not formed by a flexible hydrocarbon chain but by a rigid hydrocarbon cage.
Amphiphilic molecules are known to show a rich aggregation behavior in solution, driven by the tendency to avoid unfavorable contacts of the solvent (e.g., water) with the “wrong” parts of the amphiphilic molecule (e.g., the hydrophobic parts for aqueous solution). In chain-based surfactants and lipids, this problem is solved by aggregation into bilayer membranes that, furthermore, avoid unfavorable open edges by closing into spherical vesicles. Curiously, exactly the same aggregation behavior into spherical bilayer membranes is observed for fullerene surfactants.
The aggregates being investigated here have sizes between 10 and 100 nm, so that static and dynamic light scattering are the predominant experimental techniques to study their aggregation behavior in solution and its temporal evolution during complexation experiments. Static light scattering directly probes the size of the aggregates in terms of their radius of gyration and also provides their (weight average) molecular weight. Dynamic light scattering indirectly generates aggregate size distributions by interpreting the translational diffusive behavior of the solved species. For both experimental techniques, care must be taken that certain preconditions are met, in particular those pertaining to the non-interaction of the particles.
Complexes between cationic fullerene surfactants and DNA are promising candidates for gene delivery applications. The primary task to be solved for these applications consists of transporting the DNA through the cell membrane (“transfection”). DNA is a highly hydrophilic polyelectrolyte that prefers an open extended coil conformation in the aqueous media present at both sides of the cell membrane. The cell membrane is essentially a phospho-lipid bilayer having a hydrophobic core that is the primary barrier for DNA transfection.
The ideal way to achieve this task is demonstrated by nature in form of viruses: DNA’s extended coil conformation is condensed into a compact globular shape and encapsulated into a shell. Often there are also docking compounds for specific membrane proteins that further facilitate the penetration of the cell membrane. Despite extensive research in this direction, it has proven extremely difficult to mimic even parts of this process. In particular, the condensation of the DNA into compact globular form needs to be carried out in a way that is acceptable for therapeutic applications; e.g., any organic solvents used in the process need to be removed, and this removal must be carried out in a way that does not immediately unfold the compact globule, which turns out to be difficult.
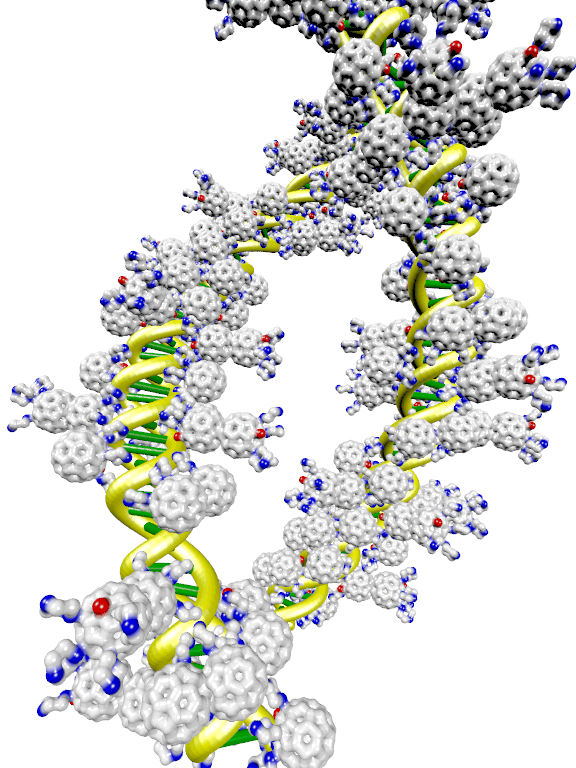
In the present project, we went a slightly different route, not requiring a full condensation of the DNA into a compact globule, but merely decorating the DNA with a compatibilizing fullerene surfactant layer (see Figure 1) while retaining a mostly open coil conformation. There is some evidence that this “stabilized” DNA can also achieve an increased transfection efficiency, while avoiding many of the problems incurred by trying to reproduce the viral route of full DNA compaction. For details, please see the references.