
ACS PRF | ACS
All e-Annual Reports

41920-AC3
Biomimetic and Bionic Catalysts for Selective Diene Hydroperoxidation
During the third (no cost, final) project year PRF support has aided our efforts to produce synthetic mimics of the iron lipoxygenase enzymes, which catalyze extraordinary regio- and stereoselective oxidation reactions. 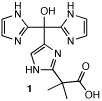
 GRA Jerome Volkman has prepared the first synthetic tris(imidazolyl)(CO2) ligands (1), which possesses the donor set of the enzyme. Several metal complexes of 1 have also been prepared, including the cobalt derivative 2, which provides the first detailed structure of a synthetic complex of the biomimetic ligand.1 Reactivity studies of Cu-, Mn- and Fe- complexes of 1 are planned for the near future to evaluate their potential as catalysts of selective hydroperoxidation. A new methodology for functionalizing these ligands to enable their covalent attachment to proteins, for producing novel, semi-synthetic selective oxidation catalysts has also been developed.2
GRA Jerome Volkman has prepared the first synthetic tris(imidazolyl)(CO2) ligands (1), which possesses the donor set of the enzyme. Several metal complexes of 1 have also been prepared, including the cobalt derivative 2, which provides the first detailed structure of a synthetic complex of the biomimetic ligand.1 Reactivity studies of Cu-, Mn- and Fe- complexes of 1 are planned for the near future to evaluate their potential as catalysts of selective hydroperoxidation. A new methodology for functionalizing these ligands to enable their covalent attachment to proteins, for producing novel, semi-synthetic selective oxidation catalysts has also been developed.2
1) Jerome Volkman and Kenneth M. Nicholas, "Synthesis of tris(imidazole)(carboxylate) ligands possessing the active site donor atom set of plant lipoxygenase", manuscript in preparation, 2007. 2) Jerome Volkman, Cecile Ouairy and K.M. Nicholas, "A new method for conjugating tertiary alcohols to proteins", manuscript in preparation, 2007.