www.acsprf.org
Reports: ND148845-ND1: Synthesis and Development of Novel Bis-Siloxy Arenes for Functionalized Magnetic Nanoparticles
David A. Spivak, PhD , Louisiana State University
1. Model study of bis-silylarenes:
Further testing on the stability of the different siloxane coatings shown in Table 1 was carried out; rates of removal of the coating by hydrolysis were determined (Table 1). Briefly, a sample of particles to be tested was suspended in dry THF and the resulting suspension distributed between multiple vials. Each vial was then prepared to give a 50% aqueous THF solution, followed by mechanical shaking at constant temperature. At predetermined times the
Table 1. Silane structures and stability data.
Compound #
| Name
| Structure
| Thermal desorption point °C
| Total loading g/g
| Initial slope of hydrolysis
| ||
1
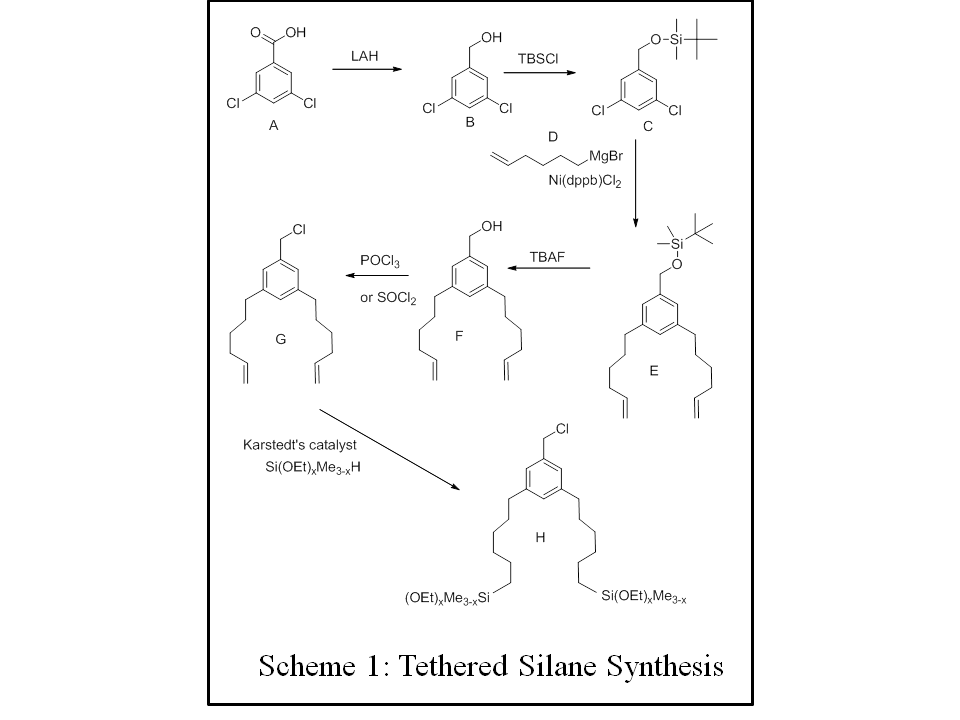
| Triethoxy-phenylsilane
|
| 384-398
| .04516
| -.005985
| ||
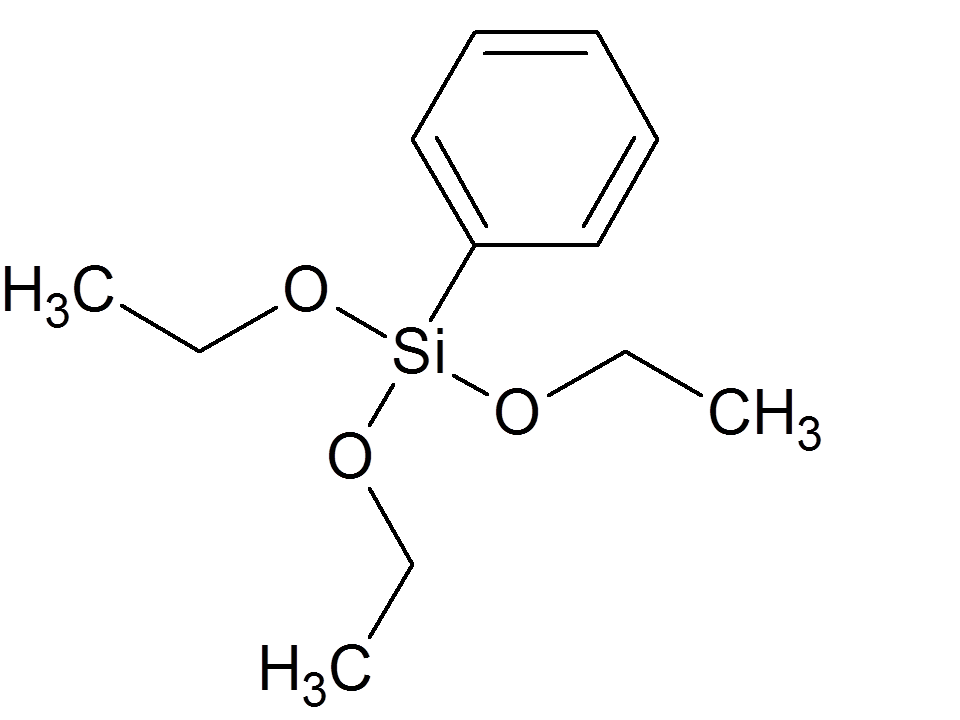
2
| Diethoxy-methyl-phenylsilane
|
| 381
| No Data
| No Data
| ||
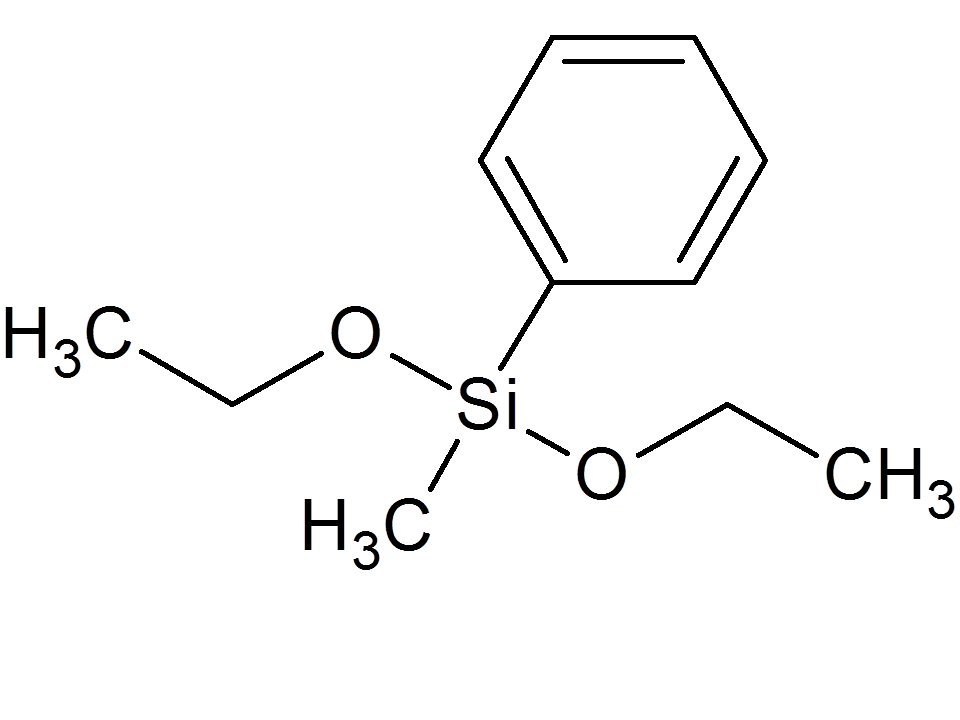
3
| Ethoxydimethyl-phenylsilane
|
| 387-389
| .09262
| -.006916
| ||
4
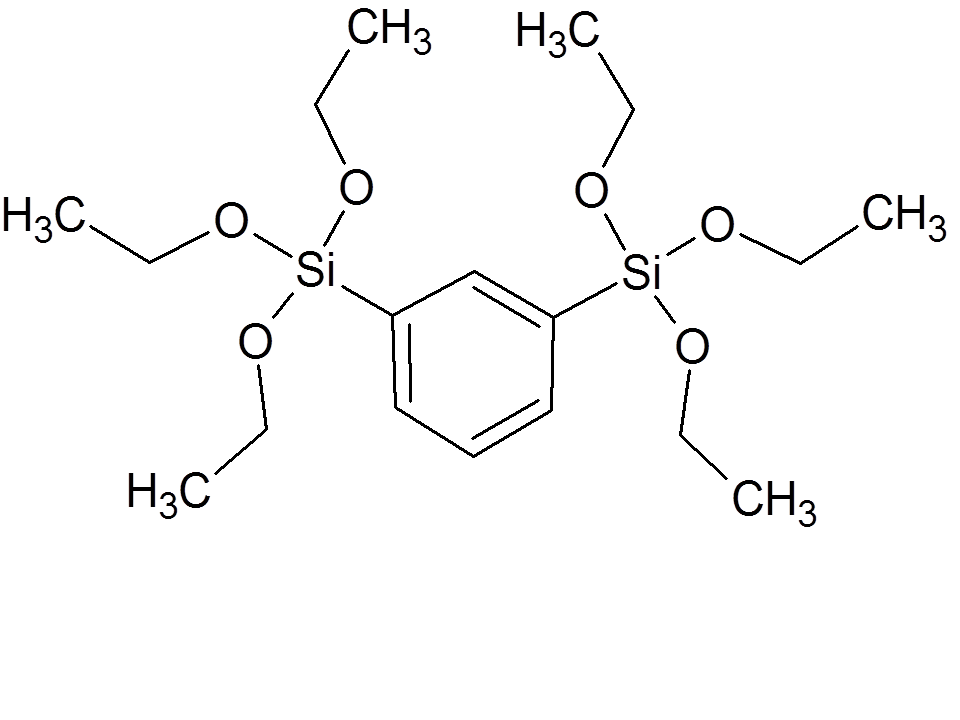
| 1,3-bis[triethoxy-silyl]benzene
|
| 396-406
| .04308
| -.006268
| ||
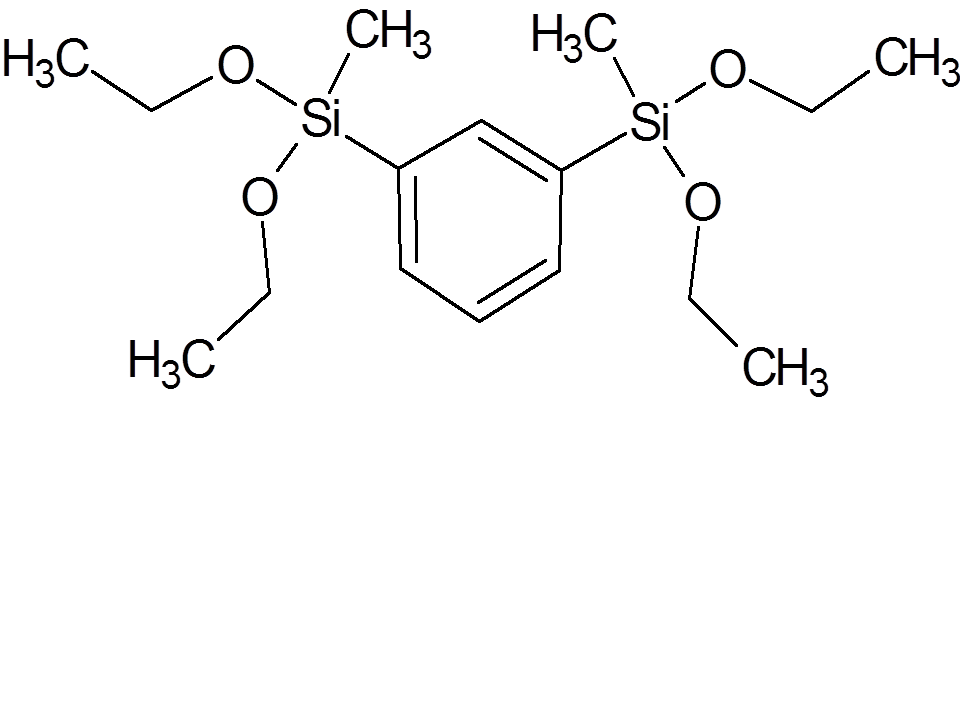
5
|
|
| 380-393
| .08255
| .000368
| ||
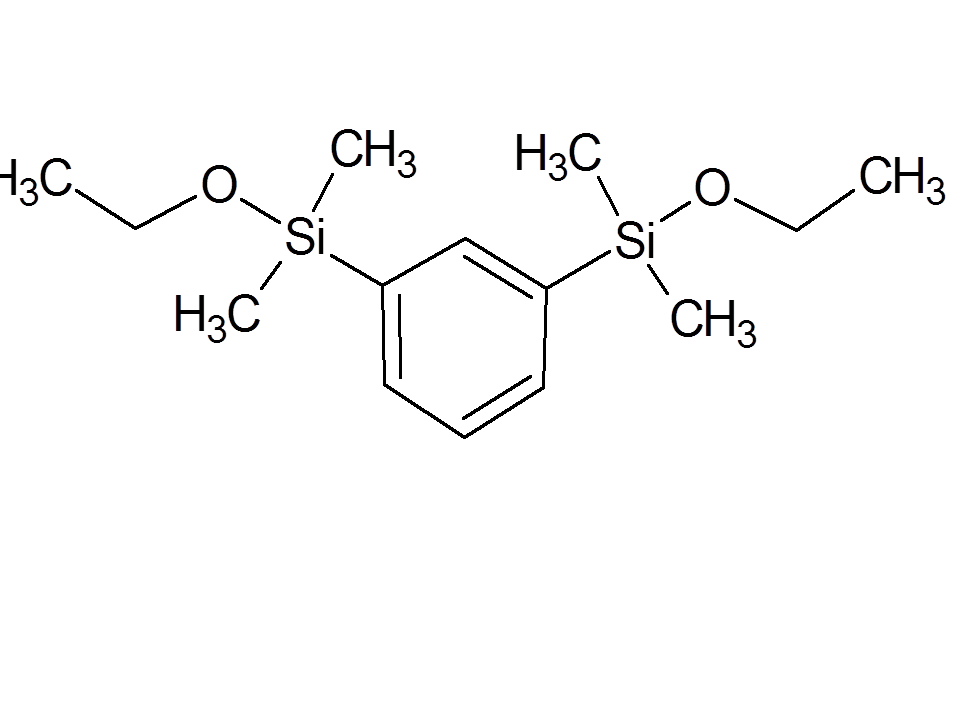
6
|
|
| 382-389
| .05579
| -.00181
|
|
|
 vials
were removed from heat and the particles cleaned and dried; then TGA was used
to determine the amount of coating remaining.
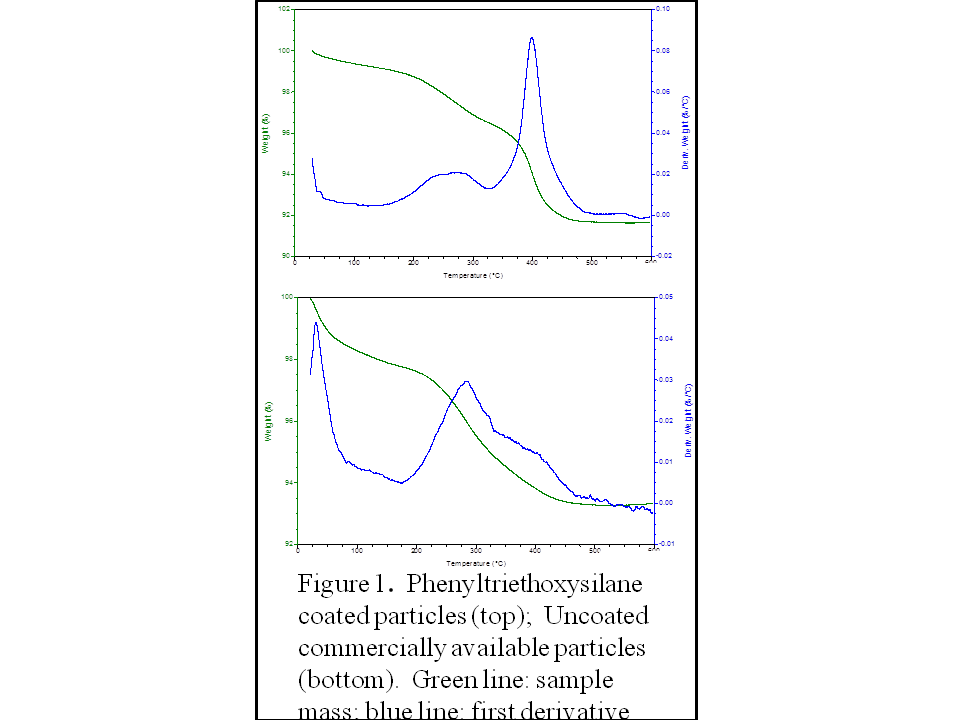
The mass of covalently bound material was determined by measuring the
mass loss in the covalent desorption region of a TGA, which is most easily
observed by looking at the first derivatives of the mass loss (Figure 1). The use of THF serves dual purposes; most
importantly it helps the aqueous solution wet the particle surface allowing
hydrolysis to occur on hydrophobic coatings[20]. Additionally, the use of dry THF to initially
suspend the particles permits the use of heating and sonication to break apart
aggregates without any aqueous hydrolysis.
vials
were removed from heat and the particles cleaned and dried; then TGA was used
to determine the amount of coating remaining.
The mass of covalently bound material was determined by measuring the
mass loss in the covalent desorption region of a TGA, which is most easily
observed by looking at the first derivatives of the mass loss (Figure 1). The use of THF serves dual purposes; most
importantly it helps the aqueous solution wet the particle surface allowing
hydrolysis to occur on hydrophobic coatings[20]. Additionally, the use of dry THF to initially
suspend the particles permits the use of heating and sonication to break apart
aggregates without any aqueous hydrolysis. During hydrolysis studies the amount of covalently bound material relative to the mass of the particles was observed to decrease as hydrolysis progressed. The first important observation is that the bis-arylsilanes are generally more stable than the mono-subsituted analogs (Table 1). A second important and surprising observation is that while compound 4 was expected to exhibit superior performance compared to other compounds due because the increase in the number of siloxane groups allows for more crosslinking within the coating, the compound in entry 5 was actually the most stable to hydrolysis! The bis-diethoxymethyl analog (entry 5) proved to be the most resistant to hydrolysis, and showed essentially no coating loss over the week long length of the experiment. The reason for this counterintuitive result is not fully understood, but the difference is significant. This ability of triethoxy compounds such as compound 4 to crosslink also can promote the formation of multilayers; therefore, it is possible that we are observing the degradation of a less stable multilayer.
2. Synthesis of tethered bis-silylarenes. Another class of bis-silylarenes is under investigation where the siloxy groups are placed at the end of a tether attached to the benzene (e.g. Scheme 1). The addition of short and medium length alkyl chains to link the aromatic core to the silane functionality may give superior coating density and stability. Synthesis of the first derivative is shown in Scheme 1. Here, we are also incorporating a third functional group (chloromethylstyrene) as a handle for further modification once the siloxane coating is achieved. In the synthesis in Scheme 1, compound C will undergo Kumada coupling with Grignard D to introduce the linking chains. The compound will then be deprotected and chlorinated to produce a benzylic halide. Hydrosylilation with Karstedt's catalyst is anticipated to give the final product. Silane films using this new class of bis-silylarenes will be tested for hydrolytic stability in a manner similar to that used for magnetite particles.
Future Work.
An important study to undertake concurrently with the studies above, is to test stability of the bis-silylarenes on glass (silicate) substrates, similar to the studies done on MNPs. Initially this work will be carried out on flat glass slides, and later evaluated on glass beads. The addition of an alkyl chain at a third position on the benzene scaffold will mimic traditional octadecylsilane surface coating, and the stability would be compared to existing materials. For flat substrates, both water contact angle and XPS measurements will be used to confirm the retention of the silane coating after exposure to hydrolytic conditions. Future work will continue to focus on methods for increasing the stability of coatings.
Another goal is to synthesize an analog of compound H incorporating a chloromethyl substituent at the 5 position on the aromatic core. Such a functional group is the same as that found in Merrifield resin and would allow these materials to act as linkers for additional surface modification.
Once the best bis-silylarene coating is found, we intend to use these linkers to attach Merrifield resin to MNPs. The resulting material would be much like the MNP/oligomer hybrids we have already produced but with the benefit of increased stability.
|
|