www.acsprf.org
Reports: B148367-B1: Beta-Hydroxysalicylhydrazones: Chiral, Non-Racemic Tridentate Catalysts for Asymmetric Synthesis
Shawn R. Hitchcock , Illinois State University
In the final granting period of this grant, our research became focused on a variety of projects that grew from our pilot efforts with the development of chiral, non-racemic ligands for the asymmetric addition of diethylzinc to aldehydes and imines. In the granting period prior to the final period, we had concluded our efforts on the synthesis of the calcimimetic agent NPS R-586 via a diethylzinc promoted asymmetric additions. As this work came to a close, it inspired a number of projects that our research pursued in the last year of the grant. These projects included work on the development of acyl succinimides as acyl transfer agents and as tools for a one-pot coupling/reduction pathway to form amines. In conjunction with this work we also pursued the synthesis of the calcimimetic agent cinacalcet using the acyl succinimide chemistry. These two projects are described here.
Project 1
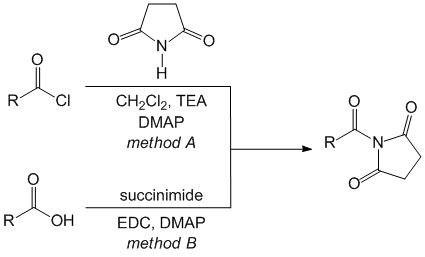
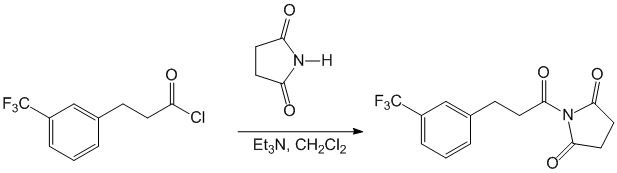
We launched our efforts in the area of acyl succinimides with the synthesis of the N-acyl succinimides was conducted by acylation of succinimide with an acyl chloride and DMAP in a dichloromethane solution with triethylamine at low temperature (method A, Scheme 1).
Scheme 1. Synthesis of N-acyl succinimides.
These conditions afforded N-acyl succinimides and N-carbamoyl succinimides in isolated yields that ranged from 56-97% (Table 1). All succinimides were obtained as crystalline solids. We also pursued the direct conversion of carboxylic acids to their corresponding N-acyl succinimides by reaction with EDC, DMAP and succinimide (method B). Using these conditions, N-benzoyl succinimide was synthesized in 56% yield after purificiation, and N-phthaloylglycyl succinimide was prepared in 38% yield.
Table 1. Synthesis of N-acyl and N-carbamoyl succinimides.
entry
| R
| method
| yielda (%)
| mp (oC)
|
1
| CH3CH2-
| A
| 56
| 34-36
|
2
| C6H5-
| A
| 66
| 122-124
|
3
| C6H5-
| B
| 56
| 128-130
|
4
| p-NO2C6H4-
| A
| 53
| 55-58
|
5
| phthaloylglycine
| B
| 38
| 223 (dec.) |
6
| t-BuO- | A
| 97
| 82-84
|
7
| C6H5CH2O-
| A
| 79
| 185-187
|
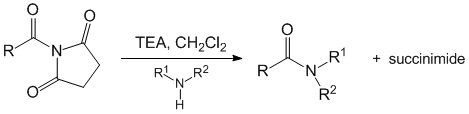
The succinimides were reacted with a variety of amines (primary, secondary, aromatic) with triethylamine at room temperature to form amides in yields ranging from 35 to 94% (Table 2). Reactions with primary amines, including sec-butylamine and tert-butylamine, afforded the corresponding amides in good yield. The use of N-propanoyl to acylate p-anisidine met with limited success with an isolated yield of 35%. In contrast, the use of N-benzoyl succinimide to prepare the benzamide of p-anisidine afforded a yield of 91%. Ultimately, this would suggest that the acyl transfer process can be made to be chemoselective.
Table 2. Acylation with N-acyl succinimides.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
| C6H5-
|
|
|
|
|
| C6H5-
|
|
|
|
|
| C6H5-
|
|
|
|
|
| C6H5-
|
|
|
|
|
| C6H5-
| |
|
|
|
| p-NO2C6H4-
|
|
|
|
|
| p-NO2C6H4-
|
|
|
|
|
| p-NO2C6H4-
|
|
|
|
|
| p-NO2C6H4-
|
|
|
|
|
| p-NO2C6H4-
|
|
|
|
There was also an interest in determining the efficiency of the acyl transfer process using the N-carbamoyl succinimides as transfer agents. The N-carbamoyl succinimides were reacted with amines using the same conditions that had been developed for the acyl transfer reactions of the N-acyl succinimides (Table 3). The purified yields of the resultant carbamates were lower than those of the amides. This may be the result of lower reactivity of the carbonyl of the carbamoyl group bound to the succinimide.
Table 3. Acylation with N-carbamoyl succinimides.
|
|
|
|
|
|
|
| |
|
|
|
|
| |
|
|
|
|
| |
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
| C6H5CH2O-
|
|
|
|
|
| C6H5CH2O-
|
|
|
|
|
| C6H5CH2O-
|
|
|
|
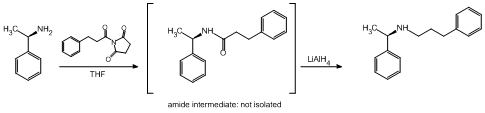
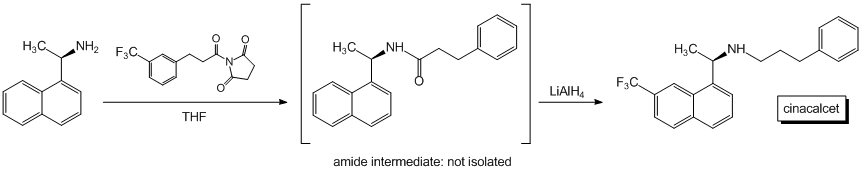
An additional reaction that we have conducted is a one pot coupling/reduction pathway using acyl succinimides that has given promising preliminary results (Scheme 2). The yield for this process is 57% and remains to be optimized. Nonetheless, we were convinced that this reaction will prove to be of interest to the synthetic community and we became interested in applying this chemistry to the synthesis of the calcimimetic agent cinacalcet.
Scheme 2. A one-pot coupling/reduction pathway.
Project 2: An efficient synthesis of the calcimimetic agent, Cinacalcet
Calcimimetic agents are medicinal agents used to treat hyperparathyroidism. There are two types of hyperparathyroidism, primary and secondary. Hyperparathyroidism can be treated with calcimimetic agents. We became interested in determining if it would be possible to develop a highly efficient synthesis of the calcimimetic agent cinacalcet. We launched our study with the synthesis of the racemic mixture as an analytical standard. In this context, we have conducted a synthesis of racemic cinacalcet. Thus, 1-naphthylethylamine was coupled with 3-(3-trifluoro methylphenyl)propenoic acid via a DCC coupling in 47% yield. The resultant amide was then hydrogenated over palladium on carbon to afford the reduced product in 64% yield. Reaction of the amide with diisobutylaluminum hydride yielded the target cinacalcet amine in 68% yield. The racemic synthesis is short and efficient, but we were interested in developing an even more convergent synthesis of the enantiomerically pure material. We opted for a methodology that would allow for the coupling and the reduction to take place in a single vessel, rather than carrying the reaction out in multiple stages. We have developed an efficient one-pot pathway for the synthesis of cinacalcet that employs the use of an easily prepared acyl succinimide from commercially available starting materials and (R)-1-naphthylethylamine. Preliminary results suggest that the single pot method will prove to be more efficient than the multiple reaction pathway (Scheme 3).
Scheme 3. Preliminary synthetic plan for the synthesis of cinacalcet.