www.acsprf.org
Reports: DNI150245-DNI1: Transition Metal Catalyzed [2+2+2] Cycloadditions for Carbocycle Synthesis
Erik J. Alexanian , University of North Carolina (Chapel Hill)
 The development of
fundamentally new complexity-increasing reactions is a paramount goal of modern
organic synthesis, as chemists strive to develop step-economical or "greener"
syntheses of complex targets. Transition-metal-catalyzed cycloadditions
of simple p-systems are examples of
powerful synthetic transformations capable of directly constructing diverse
cyclic structures from readily available starting materials. In particular,
multicomponent [m+n+o]-type
cycloadditions offer the potential for the rapid generation
of molecular complexity through the formation of multiple s-bonds and sp3-hybridized stereocenters in a single reaction step. We have initiated
the development of new classes of complexity-generating
transition-metal-catalyzed [2+2+2] cycloadditions of
easily accessible p-systems delivering
complex carbocycles for applications in chemical
synthesis.
The development of
fundamentally new complexity-increasing reactions is a paramount goal of modern
organic synthesis, as chemists strive to develop step-economical or "greener"
syntheses of complex targets. Transition-metal-catalyzed cycloadditions
of simple p-systems are examples of
powerful synthetic transformations capable of directly constructing diverse
cyclic structures from readily available starting materials. In particular,
multicomponent [m+n+o]-type
cycloadditions offer the potential for the rapid generation
of molecular complexity through the formation of multiple s-bonds and sp3-hybridized stereocenters in a single reaction step. We have initiated
the development of new classes of complexity-generating
transition-metal-catalyzed [2+2+2] cycloadditions of
easily accessible p-systems delivering
complex carbocycles for applications in chemical
synthesis.
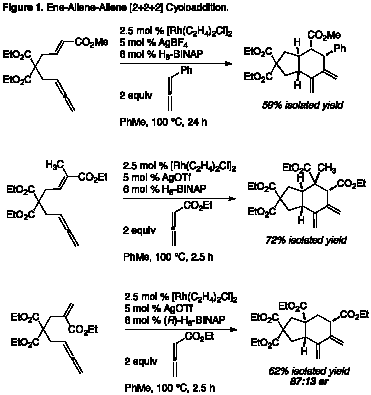
Our studies have begun with the development of a rhodium-catalyzed [2+2+2] cycloaddition of ene-allenes with allene p-systems (Figure 1). This reaction delivers synthetically valuable carbocycles and constructs up to four contiguous stereogenic centers in a single step with high stereoselectivity. The trans-hydrindane framework readily accessed via this cycloaddition is a core structure in many bioactive small molecules, yet still presents a formidable synthetic challenge. Substitution of reaction p-components leads to a variety of bicyclic compounds, including structures containing all-carbon quaternary centers. Preliminary studies have also indicated the potential for a catalytic asymmetric variant of the ene-allene-allene [2+2+2]. The development of a general, highly enantioselective process is a focus of ongoing studies.
Catalytic [2+2+2] cycloadditions which are capable of forging multiple bonds and sp3 stereocenters are rare, despite the impressive synthetic potential of such processes. These complexity-generating reactions construct important classes of fused carbocycles with levels of efficiency unmatched by current synthetic protocols, providing fundamentally new strategic choices for the rapid construction of stereochemically-dense carbocycles, including those present in many classes of bioactive, complex natural products and small molecules. We would like to thank the ACS PRF for their generous support of this project.
