www.acsprf.org
Reports: DNI348999-DNI3: Can Vanadium Pyrophosphate Coordination Complexes Perform Butane Oxidation?
Robert P. Doyle, PhD , Syracuse University
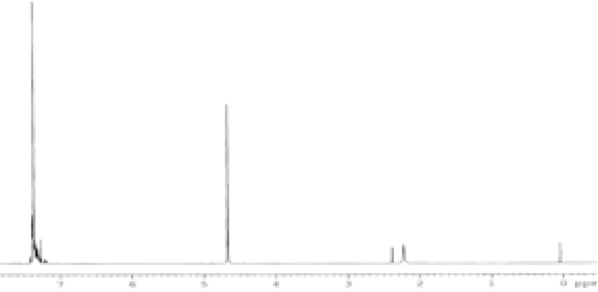
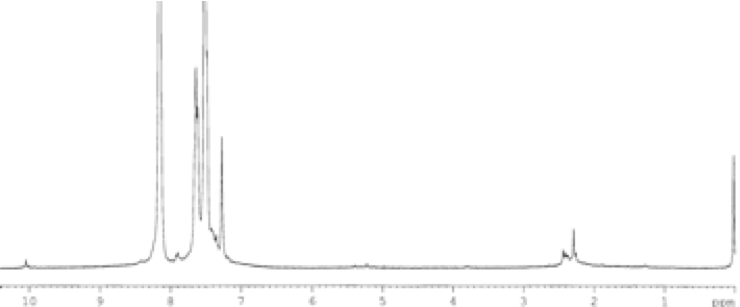
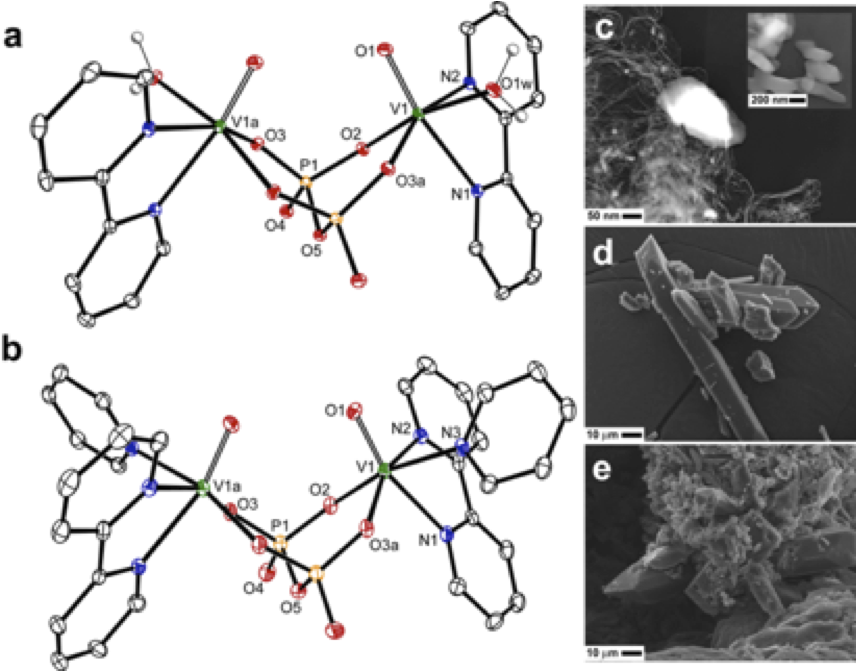

 (b)
(b)

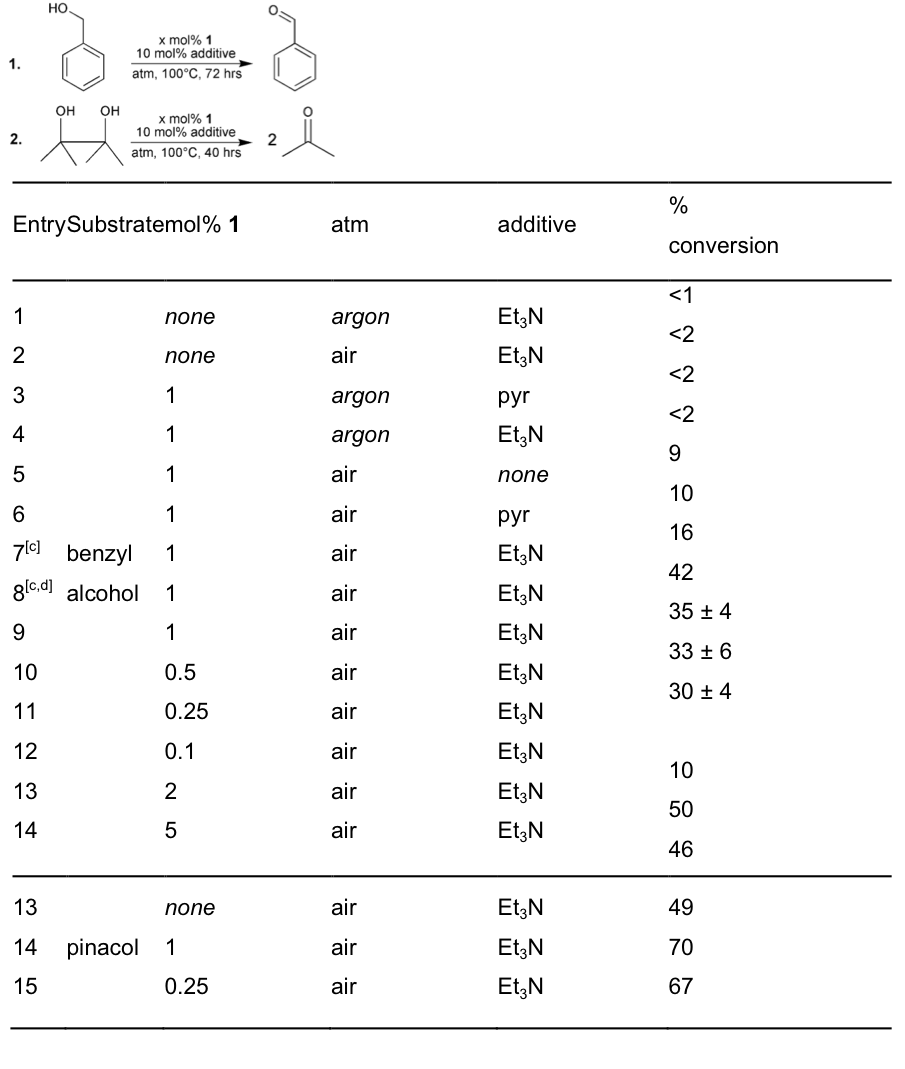 (c)
(c)
